Question 1.
What is meant by ‘lanthanoid contraction’? (Delhi 2011)
Answer:
The steady decrease in the ionic radius from La3+to Lu3+ is termed as lanthanoid contraction.
Question 2.
Why do transition elements show variable oxidation states? (Comptt. Delhi 2014)
Answer:
The variability of oxidation state of transition elements is due to incompletely filled d-orbitals and presence of unpaired electrons, i.e. (ns) and (n -1) d electrons have approximate equal energies.
Question 3.
Write the formula of an oxo-anion of Manganese (Mn) in which it shows the oxidation state equal to its group number. (Delhi 2017)
Answer:
Permanganate ion, i.e., MnO4– with oxidation number +7.
Question 4.
What happens when (NH4)2Cr2O7 is heated? (Delhi 2017)
Answer:![]()
Question 5.
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number. (Delhi 2017)
Answer:
Cr2O72- (dichromate ion) in which oxidation state of Cr is +6 which equal to its group number 6.
The d- and f-Block Elements Class 12 Important Questions Short Answer Type -I [SA – I]
Question 6.
Explain the following observations :
(i) Generally there is an increase in density of elements from titanium (Z = 22) to copper (Z = 29) in the first series of transition elements.
(ii) Transition elements and their compounds are generally found to be good catalysts in chemical reactions. (Delhi 2010)
Answer:
(i) From titanium to copper the atomic size of elements decreases and mass increases as a result of which density increases.
(ii) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d- orbitals and variable oxidation states.
Question 7.
Explain the following observations :
(i) Transition elements generally form coloured compounds.
(ii) Zinc is not regarded as a transition element. (Delhi 2010)
Answer:
(i) Because of presence of unpaired d electrons, which undergoes d-d transition by absorption of energy from visible region and then the emitted light shows complementary colours. This is how transition elements form coloured compounds.
(ii) Zinc in its common oxidation state of +2 has completely filled d-orbitals. Hence considered as non-transition element.
Question 8.
Assign reasons for the following :
(i) Copper (I) ion is not known in aqueous solution.
(ii) Actinoids exhibit greater range of oxidation states than lanthanoids. (Delhi 2011)
Answer:
(i) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but Δhyd (hydration enthalpy) for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows :
2Cu+ → Cu2+ + Cu
(ii) Because of very small energy gap between 5f, 6d and 7s subshells all their electrons can take part in bonding and shows variable oxidation states.
Question 9.
Assign reasons for each of the following :
(i) Transition metals generally form coloured compounds.
(ii) Manganese exhibits the highest oxidation state of +7 among the 3d series of transition elements. (Delhi 2011)
Answer:
(i) Because presence of unpaired d electrons, which undergoes d-d transition by absorption of energy from visible region and then the emitted light shows complementary colours.
(ii) Manganese exhibits highest oxidation of +7 among 3d series of transition elements because all the oxidation states are exhibited from +2 to +7 by Mn and no other element of this series shows this highest state of oxidation.
Question 10.
How would you account for the following :
(i) Cr2+ is reducing in nature while with the same d-orbital configuration (d4) Mn3+ is an oxidising agent.
(ii) In a transition series of metals, the metal
which exhibits the greatest number of oxidation states occurs in the middle of the series. (All India 2011)
Answer:
(i) Cr2+ has the configuration 3d4 which easily changes to d3 due to stable half filled t2g orbitals. Therefore Cr2+ is reducing agent. While Mn2+ has stable half filled d5 configuration. Hence Mn3+easily changes to Mn2+ and acts as oxidising agent.
(ii) Due to presence of more unpaired electrons and use of all 4s and 3d electrons in the middle of series.
Question 11.
Complete the following chemical equations : (All India 2011)
(i) MnO4 (aq) + S2O32- (aq) + H2O (1) →
(ii) Cr2O72- (aq) + Fe2+ (aq) + H+ (aq) →
Answer: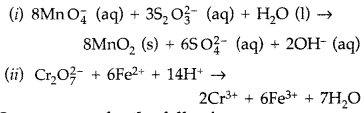
Question 12.
State reasons for the following :
(i) Cu (I) ion is not stable in an aqueous solution.
(ii) Unlike Cr3+, Mn2+, Fe3+ and the subsequent other M2+ ions of the 3d series of elements, the 4d and the 5d series metals generally do not form stable cationic species. (All India 2011)
Answer:
(i) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but Δhyd (hydration enthalpy) for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows :
2Cu+ → Cu2+ + Cu
(ii) Because high enthalpies of atomisation of 4d and 5d series and high ionization enthalpies, the M.P. and B.P. of heavier transition elements are greater than those of first transition series which is due to stronger intermetallic bonding. Hence 4d and 5d series metals generally do not form stable cationic species.
Question 13.
Explain giving a suitable reason for each of the following :
(i) Transition metals and their compounds are generally found to be good catalysts.
(ii) Metal-metal bonding is more frequent for the 4d and the 5d series of transition metals than that for the 3d series. (All India 2011)
Answer:
(i) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d- orbitals and variable oxidation states.
(ii) Metal-metal bonding is more frequent for the 4d and the 5d series of transition metals than that for the 3d series as these have their electrons of outer most shell at greater distance from the nucleus, as compared to atoms of 3d transition metals.
Question 14.
Explain giving reasons :
(i) Transition metals and their compounds generally exhibit a paramagnetic behaviour.
(ii) The chemistry of actinoids is not so smooth as that of lanthanoids. (All India 2011)
Answer:
(i) Because of presence of unpaired electrons in their d-subshell in atomic and ionic state.
(ii) Lanthanoids show limited number of oxidation state, viz. +2, +3 and +4 (out of which +3 is most common) because of large energy gap between 4f and 5d subshells. Actinoids also show stable +3 oxidation state but show a number of oxidation states i.e. +4, +5 and + 6, +7 due to small energy difference between 5f, 6d and 7s subshells.
Question 15.
Complete the following chemical equations : (Delhi 2012)![]()
Answer: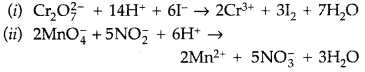
Question 16.
Explain the following :
(a) The enthalpies of atomization of transition metals are quite high.
(b) The transition metals and many of their compounds act as good catalysts. (Comptt. Delhi 2012)
Answer:
(a) In transition elements, there are large number of unpaired electrons in their atoms, thus they have a stronger inter atomic interaction and thereby stronger bonding between the atoms. Due to this they have high enthalpies of atomization.
(b) Because of the availability of d-orbitals, they can easily form intermediate products which are activated. The sizes of transition metal atoms and ions are also favourable for transition complex formation with the reactants.
Question 17.
Complete the following chemical reaction equations : (All India 2012)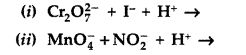
Answer: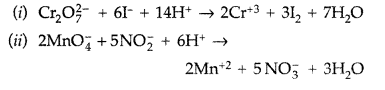
Question 18.
(a) Which metal in the first transition series (3d series) exhibits +1 oxidation state most frequency and why?
(b) Which of the following cations are coloured in aqueous solutions and why?
SC3+, V3+, Ti4+, Mn2+.
(At. nos. Sc = 21, V = 23, Ti = 22, Mn = 25) (Delhi 2013)
Answer:
(a) Copper exhibits + 1 oxidation state more frequently i.e., Cu+1 because of its electronic configuration 3d104s1. It can easily lose 4s1electron to give stable 3d10 configuration.
(b) SC3+ = 4S0 3d3+ = no unpaired electron
V3+ = 3d2 4s0 = 2 unpaired electron
Ti4+ = 3d0 4s0 = no unpaired electron
Mn2+ = 3d5 4s0 = 5 unpaired electron
Thus V3+ and Mn2+ are coloured in their aqueous solution due to presence of unpaired electron.
Question 19.
What is Lanthanoid contraction? What are its two consequences? (Comptt. Delhi 2013)
Answer:
Lanthanoid contraction: The overall decrease in atomic and ionic radii with increasing atomic number is known as lanthanoid contraction. In going from La+3 to Lu+3 in lanthanoid series, the size of ion decreases. This decrease in size in the lanthanoid series is known as lanthanoid contraction. The lanthanoid contraction arises due to imperfect shielding of one 4f electron by another present in the same subshell.
Consequences :
(i) Similarity in properties: Due to lanthanoid contraction, the size of elements which follow (Hf – Hg) are almost similar to the size of the elements , of previous row (Zr – Cd) and hence these are difficult to separate. Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoid becomes very difficult.
(ii) Basicity difference : Due to lanthanoid contraction, the size decreases from La+3 to Lu+3. Thus covalent character increases. Hence basic character of hydroxides also decreases i.e. why La(OH)3 is most basic while Lu(OH)3 is least basic.
Question 20.
Assign a reason for each of the following observations:
(i) The transition metals (with the exception of Zn, Cd and Hg) are hard and have high melting and boiling points.
(ii) The ionization enthalpies (first and second) in the first series of the transition elements are found to vary irregularly. (Comptt. Delhi 2014)
Answer:
(i) Because of stronger metallic bonding and high enthalpies of atomization.
(ii) Due to irregulaties in the electronic configuration there is irregularities in the enthalpies of atomisation. Hence there is irregular variation in I.E.
Question 21.
What is lanthanoid contraction? Write a conse-quence of lanthanoid contraction. (Comptt. Delhi 2014)
Answer:
Lanthanoid contraction: The overall decrease in atomic and ionic radii with increasing atomic number is known as lanthanoid contraction. In going from La+3 to Lu+3 in lanthanoid series, the size of ion decreases. This decrease in size in the lanthanoid series is known as lanthanoid contraction. The lanthanoid contraction arises due to imperfect shielding of one 4f electron by another present in the same subshell.
Consequences :
(i) Similarity in properties: Due to lanthanoid contraction, the size of elements which follow (Hf – Hg) are almost similar to the size of the elements , of previous row (Zr – Cd) and hence these are difficult to separate. Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoid becomes very difficult.
(ii) Basicity difference : Due to lanthanoid contraction, the size decreases from La+3 to Lu+3. Thus covalent character increases. Hence basic character of hydroxides also decreases i.e. why La(OH)3 is most basic while Lu(OH)3 is least basic.
Question 22.
How would you account for the following?
(i) The highest oxidation state of a transition metal is usually exhibited in its oxide.
(ii) The oxidising power of the following three oxoions in the series follows the order: (Comptt. Delhi 2014)
Answer:
(i) The highest oxidation state of a metal is exhibited in its oxide or fluoride due to its high electronegativity, low ionisation energy and small size.
(ii) La3+ (Z = 57) and Lu3+ (Z = 71) do not show any colour in solutions.
Question 23.
Assign reason for each of the following :
(i) Transition elements exhibit paramagnetic behaviour.
(ii) Co2+. is easily oxidised in the presence of a strong ligand. (Comptt. Delhi 2014)
Answer:
(i) Because of presence of unpaired electrons in their d-subshell in atomic and ionic state.
(ii) Co2+ ion is easily oxidised to Co3+ ion in presence of a strong ligand because of its higher crystal field energy which causes pairing of electrons to give inner orbital complexes (d2sp3).
Question 24.
Describe the general trends in the following properties of the first series (3d) of the transition elements :
(i) Number of oxidation states exhibited
(ii) Formation of oxometal ions (Comptt. Delhi 2014)
Answer:
(i) The number of oxidation states increases upto middle of series i.e. unto +7 and then decreases.
(ii) Oxometal ions are polyatomic ions with oxygen.
Example : VO2+, VO+2, T1O2+
Question 25.
Assign reasons for the following :
(i) Copper(I) ion is not known to exist in aqueous solutions.
(ii) Both O2 and F2 stabilize high oxidation states of transition metals but the ability of oxygen to do so exceeds that of fluorine. (Comptt. All India 2014)
Answer:
(i) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but Δhyd (hydration enthalpy) for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows :
2Cu+ → Cu2+ + Cu
(ii) The ability of O2 to stabilize higher oxidation states exceeds that of fluorine because oxygen can form multiple bonds with metals.
Question 26.
Assign reasons for the following :
(i) Transition metals and many of their compounds act as good catalysts.
(ii) Transition metals generally form coloured compounds. (Comptt. All India 2014)
Answer:
(i) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d-orbitals and variable oxidation states.
(ii) Because of presence of unpaired electrons in d-orbital, which undergoes d-d transition by absorption of energy from visible region and then the emitted light shows complementary colours.
Question 27.
What are the transition elements? Write two characteristics of the transition elements. (Delhi 2015)
Answer:
Elements which have partially filled d-orbital in its ground states or any one of its oxidation states are called transition elements.
- They show variable oxidation states.
- They form coloured ions.
- They form complex compounds.
Question 28.
Write one similarity and one difference between the chemistry of lanthanoids and that of actinoids. (All India 2015)
Answer:
Similarity : Both lanthanoids and actinoids show contraction in size and irregularity in their electronic configuration.
Difference: Actinoids show wide range of oxidation states but lanthanoids do not.
Question 29.
What is meant by ‘disproportionation’? Give an example of a disproportionation reaction in aqueous solution. (Comptt. Delhi 2015)
Answer:
Disproportionation: In a disproportionation reaction an element undergoes self-oxidation as well as self-reduction forming two different compounds.![]()
Question 30.
Suggest reasons for the following features of transition metal chemistry :
(i) The transition metals and their compounds are usually paramagnetic.
(ii) The transition metals exhibit variable oxidation states. (Comptt. Delhi 2015)
Answer:
(i) The transition metals and their compounds are usually paramagnetic because of the presence of unpaired electrons in their d-orbitals.
(ii) The transition metals exhibit variable oxidation states because of very close energies of incompletely filled (n – l)d orbitals and ns orbitals due to which both can participate in bonding.
Question 31.
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with oxalic acid? Write the ionic equations for the reactions. (Comptt. All India 2015)
Answer:
Potassium Permangante (KMnO4) is prepared from pyrolusite ore (MnO2). The ore (MnO2) is fused with an alkali metal hydroxide like KOH in the presence of air or an oxidising agent like KNO3to give dark green potassium manganate (K2MnO4). K2MnO4 disproportionates in a neutral or acidic solution to give potassium permanganate.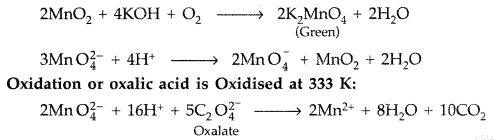
Question 32.
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with
(i) iodine (ii) H2S. (Comptt. All India 2015)
Answer:
Potassium dichromate (K2Cr2O7) acts as a strong oxidising agent in acidic medium using H2SO4.
K2Cr2O7 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O]
Ionic reactions :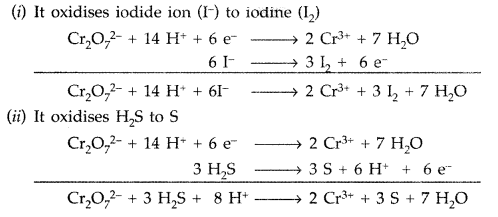
Question 33.
When chromite ore FeCr2O4 is fused with NaOH in presence of air, a yellow coloured compound (A) is obtained which on acidification with dilute sulphuric acid gives a compound (B). Compound (B) on reaction with KC1 forms an orange coloured crystalline compound (C).
(i) Write the formulae of the compounds (A), (B) and (C).
(ii) Write one use of compound (C). (Delhi 2016)
Answer:
The chromite ore FeCr2O4 on fusion with NaOH in presence of air, forms a yellow coloured compound (A) i.e. Sodium chromate.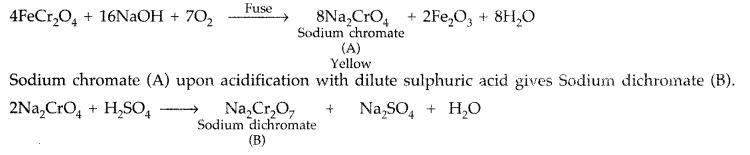
Sodium dichromate (B) on reaction with KCl forms orange coloured compound Potassium dichromate (C).
Na2Cr2O7 + 2KCl → 2NaCl + K2Cr2O7 (C)
(i) Thus (A) → Sodium chromate Na2CrO4
(A) → Sodium dichromate Na2Cr2O7
(B) → Potassium dichromate K2Cr2O7
(ii) (C) is used as a strong oxidising agent in acidic medium in volumetric analysis.
Question 34.
Complete the following chemical equations: (Delhi 2016)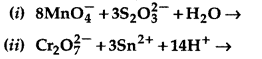
Answer: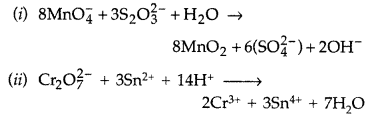
Question 35.
Give reasons :
(i) Transition metals show variable oxidation, states.
(ii) Actinoids show wide range of oxidation states. (Comptt. Delhi 2016)
Answer:
(i) Because in transitional elements ns and (n – 1 )d electrons have approximate equal energies.
(ii) Due to comparable energies of 5f, 6d and 7s orbitals.
Question 36.
Give reasons :
(i) Zn is not regarded as a transition element.
(ii) Cr2+ is a strong reducing agent. (Comptt All India 2016)
Answer:
(i) Zinc Atomic no. 30 have EC 3d10, 4s2. It has completely filled ‘d’ orbitals.
(ii) Cr+2 (3d4) after loosing one electron forms Cr+3(d3). It has
Or
Cr+3 is more stable than Cr+2, therefore Cr+2 looses one electron. Hence acting as strong reducing agent.
Question 37.
What happens when (NH4)2Cr2O7 is heated? (Delhi 2017)
Answer: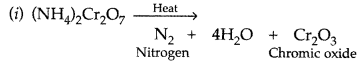
Question 38.
Explain the following observations :
(i) Copper atom has completely filled d orbitals (3d10) in its ground state, yet it is regarded as a transition element.
(ii) Cr2+ is a stronger reducing agent than Fe2+ in aqueous solutions. (Comptt. All India 2017)
Answer:
(i) Copper atom has completely filled d orbitals (3d10) in its ground state, yet it is regarded as a transition element due to incompletely filled d-orbital in its ionic states i.e. Cu2+ (3d9).
(ii) The highest oxidation state for Cr is +6, therefore it can loose 3 more electrons, whereas Fe needs to loose only 1 electron to achieve its highest oxidation state of +3. Thus, Cr3+ is more reducing than Fe2+.
Question 39.
Explain the following observations :
(a) Silver atom has completely filled d-orbitals (4d10) in its ground state, yet it is regarded as a transition element.
(b) E0 value for Mn3+ /Mn2+ couple is much more positive than Cr3+/Cr2+. (Comptt. All India 2017)
Answer:
(a) Because silver has incomplete d-orbital (4d9) in its +2 oxidation state, hence it is a transition element.
(b) The large positive E° value for Mn3+/Mn2+shows that Mn2+ is much more stable than Mn+3due to stable half filled configuration (3d5). Therefore the 3rd ionisation energy of Mn will be very high and Mn3+ is unstable and can be easily reduced to Mn2+. E° value for Cr3+ | Cr2+ is positive but small i.e. Cr3+ can also be reduced to Cr2+ but less easily. Thus Cr3+ is more stable than Mn3+.
Question 40.
Explain the following observations :
(i) Zn2+ salts are colourless.
(ii) Copper has exceptionally positive
Answer:
(i) Zn2+ salts are colourless due to absence of unpaired electrons in its ground state and ionic state i.e. Zn2+ = [Ar] 3d104s04p0
(ii) The E0M2+/M for any metal is related to the sum of the enthalpy changes taking place in the following steps :
M(g) + ΔaH → M(g) (ΔaH = enthalpy of atomization)
M(g) + ΔiH → M2+(g) (ΔiH = ionization of atomization)
M2+(g) + aq → M2+(g) + ΔhydH (ΔhydH = hydration atomization)
Copper has high enthalpy of atomization (i.e. energy absorbed and low enthalpy of hydration (i.e. energy released). Hence E0M2+/M for copper is positive. The high energy required to transform Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
The d- and f-Block Elements Class 12 Important Questions Short Answer Type -II [SA – II]
Question 41.
How would you account for the following :
(i) Many of the transition elements and their compounds can act as good catalysts.
(ii) The metallic radii of the third (5d) series of transition elements are virtually the same as those of the corresponding members of the second series.
(iii) There is a greater range of oxidation states among the actinoids than among the lanthanoids. (All India 2009)
Answer:
(i) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d-orbitals and variable oxidation states.
(ii) Due to lanthanoid contraction in second series after lanthanum, the atomic radii of elements of second and third series become almost same and hence show similarities in properties.
(iii) Because of very small energy gap between 5f, 6d and 7s subshells all their electrons can take part in bonding and shows variable oxidation states.
Question 42.
How would you account for the following?
(i) The atomic radii of the metals of the third (5d) series of transition elements are virtually the same as those of the corresponding members of the second (4d) series.
(ii) The E° value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+ couple or Fe2+/Fe2+ couple.
(iii) The highest oxidation state of a metal is exhibited in its oxide or fluoride. (Delhi 2010)
Answer:
(i) Due to lanthanoid contraction in second series after lanthanum, the atomic radii of elements of second and third series become almost same and hence show similarities in properties.
(ii) The electronic configuration of Mn2+ ion is more symmetrical as compared to that of Cr2+ ion. So 3rd ionisation potential of Mn2+ is much higher. As a result E° value of Mn3+/ Mn2+ couple is much more positive than for Cr3+/Cr2+ couple.
(iii) The highest oxidation state of a metal is exhibited in its oxide or fluoride due to its high electronegativity.
Question 43.
Complete the following chemical equations : (Delhi 2011)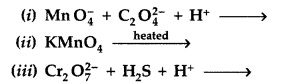
Answer: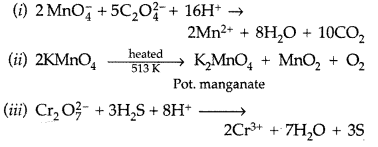
Question 44.
How would you account for the following?
(i) Many of the transition elements are known to form interstitial compounds.
(ii) The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group members of the second (4d) series.
(iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. (Delhi 2012)
Answer:
(i) The transition metals form a large number of interstitial compounds in which small atoms such as hydrogen, carbon, boron and nitrogen occupy the empty spaces in the crystal lattices of transition metals.
(ii) Because of lanthanoid contraction.
(iii) This is due to comparable energies of 5f, 6d, 7s orbitals in actinoids.
Question 45.
How would you account for the following?
(i) With the same d-orbital configuration (d4) Cr2+is a reducing agent while Mn3+ is an oxidizing agent.
(ii) The actinoids exhibit a larger number of oxidation states than the corresponding members in the lanthanoid series.
(iii) Most of the transition metal ions exhibit characteristic in colours in aqueous solutions. (Delhi 2012)
Answer:
(i) Cr2+ has the configuration 3d4 which easily changes to d3 due to stable half filled t2g orbitals. Therefore Cr2+ is reducing agent. While Mn2+ has stable half filled d5 configuration. Hence Mn3+easily changes to Mn2+ and acts as oxidising agent.
(ii) Due to comparable energies of 5f 6d and 7s orbitals of actinoids, these show larger number of oxidation states than corresponding members of lanthanoids.
(iii) Due to the presence of unpaired electrons in d-orbital, transition metal exhibits colours in aqueous solution or due to d-d transition.
Question 46.
Explain the following observations giving an appropriate reason for each.
(i) The enthalpies of atomization of transition elements are quite high.
(ii) There occurs much more frequent metal- metal bonding in compounds of heavy transition metals (i.e. 3rd series).
(iii) Mn2+ is much more resistant than Fe2+towards oxidation. (Delhi 2012)
Answer:
(i) In transition elements, there are large number of unpaired electrons in their atoms, thus they have a stronger inter atomic interaction and thereby stronger bonding between the atoms. Due to this they have high enthalpies of atomization.
(ii) Because of high enthalpy of atomisation of 3rdseries, there occurs much more frequent metal-metal bonding in compounds of heavy transition metals.
(iii) The 3d orbital in Mn2+ is half-filled and is more stable compared to Fe2+ has 6 electrons in the 3d orbital. Mn2+ prefer to lose an electron or get oxidised whereas Fe2+ will readily loose one electron or get oxidised. Therefore, Mn2+ is much more resistant than Fe2+ towards oxidation.
Question 47.
How would you account for the following :
(i) Among lanthanoids, Ln (III) compounds are predominant. However, occasionally in solutions or in solid compounds, + 2 and + 4 ions are also obtained.
(ii) The E°M2+/M for copper is positive (0.34 V).
Copper is the only metal in the first series of transition elements showing this behaviour.
(iii) The metallic radii of the third (5d) series of transition metals are nearly the same as those of the corresponding members of the second series. (All India 2012)
Answer:
(i) Among lanthanoids, Ln (III) compounds are predominant. However +2 and +4 ions in solution or in solid compounds are also obtained. This is because they have empty, half-filled and completely filled 4/ sub-shell respectively which show extra stability.
(ii) E°M2+/M for any metal is related to the sum of enthalpy of atomisation, ionization and hydration enthalpy. Copper has high enthalpy of atomisation and low enthalpy of hydration. Hence E°Cu2+/Cu is positive. The high energy required to transform Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(iii) Because of lanthanoid contraction the metallic radii of the third (5d) series of transition metals are nearly the same as those of the corresponding members of the second series.
Question 48.
Explain the following observations :
(i) Many of the transition elements are known to form interstitial compounds
(ii) There is a general increase in density from titanium (Z = 22) to copper (Z = 29).
(iii) The members of the actinoid series exhibit a larger number of oxidation states than the corresponding members of the lanthanoid series. (All India 2012)
Answer:
(i) The transition metals form a large number of interstitial compounds in which small atoms such as hydrogen, carbon, boron and nitrogen occupy the empty spaces in the crystal lattices of transition metals.
(ii) From titanium to copper the atomic size of elements decreases and mass increases as a result of which density increases.
(iii) This is due to comparable energies of 5f 6d and 7s orbitals in actinoids.
Question 49.
Explain each of the following observations :
(i) With the same d-orbital configuration (d4), Cr2+is a reducing agent while Mn3+ is an oxidising agent.
(ii) Actinoids exhibit a much larger number of oxidation states than the lanthanoids.
(iii) There is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals. (All India 2012)
Answer:
(i) Cr+2 is reducing agent as its configuration changes from d4 to d3 which is having half filled t2g level whereas Mn+3 to Mn+2 results in stable half filled d5 configuration hence it is oxidising.
(ii) Due to comparable energies of 5f, 6d and 7s orbitals actinoids exhibit a much larger number of oxidation states than the lanthanoids.
(iii) Along a transition series, the nuclear charge increases which tends to decrease the size but the addition of electrons in the penultimate d-subshell increases the screening effect which counter balances the effect of increased nuclear charge. Thus, atomic radius does not change much.
Question 50.
Give reasons :
(a) There is a gradual decrease in the size of atoms with increasing atomic number in the series of lanthanoids.
(b) Sc (21) is a transition element but Ca (20) is not.
(c) The Fe2+ is much more easily oxidised to Fe2+than Mn2+ to Mn3+. (Comptt. Delhi 2012)
Answer:
(a) This is due to lanthanoid contraction.
(b) Sc (21) is regarded as a transition element due to the presence of incomplete d- subshell (3d14s2) but Ca (20) does not have any d-subshell.
(c) Fe+2[Ar] 3d6 can be easily oxidised to Fe+3 [Ar] 3d5, which is half filled and is more stable but Mn+2 is d5 in configuration which is again half filled configuration and Mn+3 is d4 in configuration.
Question 51.
(a) What is lanthanoid contraction? Mention its main consequences.
(b) Write the balanced ionic equation for the reaction between ferrous sulphate and acidified potassium permanganate solution. (Comptt. Delhi 2012)
Answer:
(a) Lanthanoid contraction : The overall decrease in atomic and ionic radii with increasing atomic number is known as lanthanoid contraction. In going from La+3 to Lu+3 in lanthanoid series, the size of ion decreases. This decrease in size in the lanthanoid series is known as lanthanoid contraction. The lanthanoid contraction arises due to imperfect shielding of one 4f electron by another present in the same subshell.
Consequences :
(i) Similarity in properties : Due to lanthanoid contraction, the size of elements which follow (Hf – Hg) are almost similar to the size of the elements , of previous row (Zr – Cd) and hence these are difficult to separate. Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoid becomes very difficult.
(ii) Basicity difference : Due to lanthanoid contraction, the size decreases from La+3 to Lu+3. Thus covalent character increases. Hence basic character of hydroxides also decreases i.e. why La(OH)3 is most basic while Lu(OH)3 is least basic.
(b) Balanced ionic equation :
MnO4– + 5Fe+2 + 8H+ → Mn+2 + 5Fe+3 + 4H2O
Question 52.
How would you account for the following?
(i) Transition metals exhibit variable oxidation states.
(ii) Zr (Z = 40) and Hf (Z = 72) have almost identical radii.
(iii) Transition metals and their compounds act as catalyst. (Delhi 2013)
Answer:
(i) Because the energy difference between (n-1) d-orbitals and ns-orbitals is very less. Since there is very little energy difference between these orbitals, both energy levels can be used for bond formation. Thus transition elements exhibit variable oxidation states.
(ii) Zr and Hf have almost identical radii due to lanthanoid contraction which is due to weak shielding of d-electrons.
(iii) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d-orbitals and variable oxidation states.
Question 53.
Complete the following chemical equations: (Delhi 2013)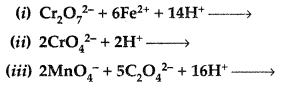
Answer: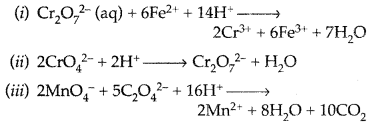
Question 54.
Write balanced chemical equations for the two reactions showing oxidizing nature of potassium permanganate. (Comptt. Delhi 2013)
Answer:
Reactions showing oxidising nature of KMnO4
(i) 2KMnO4 + 5SO2 + 2H2O → K2SO4+ 2MnSO4 + 2H2SO4
(ii) 2KMnO4 + 3H2SO4 + 5H2O → K2SO4 + 2MnSO4+ 3H2O + 5S
Question 55.
Give reasons for the following :
(i) Transition metals exhibit a wide range of oxidation states.
(ii) Cobalt(II) is very stable in aqueous solutions but gets easily oxidised in the presence of strong ligands.
(iii) Actinoids exhibit a greater range of oxidation states than lanthanoids. (Comptt. All India 2014)
Answer:
(i) The variability of oxidation state of transition elements is due to incompletely filled d-orbitals as ns, and (n – 1) d electrons have very less energy difference.
(ii) Co2+ ion is easily oxidised to Co3+ ion in presence of a strong ligand because of its higher crystal field energy which causes pairing of electrons to give inner orbital complexes (d2sp3).
(iii) Actinoids because of very small energy gap between 5f, 6d and 7s subshells all their electrons can take part in bonding and shows variable oxidation states.
Question 56.
Assign reasons for the following :
(i) Cu(I) ion is not known to exist in aqueous solutions.
(ii) Transition metals are much harder than the alkali metals.
(iii) From element to element actinoid contraction is greater than the lanthanoid contraction. (Comptt. All India 2014)
Answer:
(i) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but Δhyd (hydration enthalpy) for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows :
2Cu+ → Cu2+ + Cu
(ii) In transitional elements, in addition to metallic bonding there is extra covalent bonding due to presence of unpaired electrons in their ‘d’ orbitals, hence they are much harder.
(iii) The actinoid contraction is greater than lanthanoid contraction due to poorer shielding of 5f electrons as they are extended in space beyond 6s and 6p orbitals whereas 4f orbitals are buried deep inside the atom.
Question 57.
(a) How would you account for the following :
(i) Actinoid contraction is greater than lanthanoid contraction.
(ii) Transition metals form coloured compounds.
Complete the following equation :
2MnO4– + 6H+ + 5NO2– → (Delhi 2015)
Answer:
(a) (i) Actinoid contraction is greater than lanthanoid because 5f electrons (in actinoids) have a poorer shielding effect than 4f electrons (in lanthanoids).
(ii) Transition metals from coloured compounds because they have unpaired electrons in the d-orbital.
(b) 2MnO4– + 6H++ 5NO2 → 2Mn+2 + 5NO3– + 3H2O
Question 58.
(a) Account for the following :
(i) Cu+ is unstable in an aqueous solution.
(ii) Transition metals form complex compounds.
(b) Complete the following equation :
Cr2O72- + 8H+ + 3NO–2 → (All India 2015)
Answer:
(a) (i) Cu+ is unstable in an aqueous solution because Cu+ undergoes disproportionation reaction as follows:
2Cu+ →Cu2++ Cu
(ii) Transition metals form complex compounds due to small size of metal, higher nuclear (ionic) charge and availability of vacant or incompletely filled d-orbitals.
(b) Cr2O72- + 8H+ + 3NO–2 → 2Cr3+ + 3NO–3 + 4H2O
Question 59.
What is lanthanoid contraction? What are the consequences of lanthanoid contraction? (Comptt. Delhi 2015)
Answer:
Lanthanoid contraction: The overall decrease in atomic and ionic radii with increasing atomic number from La to Lu due to imperfect shielding of 4f-orbital is known as lanthanoid contraction. Cause : As we move along the lanthanoid series, the effective nuclear charge increases on addition of electrons and the electrons added in f-subshell causes imperfect shielding which is unable to counterbalance the effect of the increased nuclear charge. Hence the contraction in size occurs.
Consequences :
(i) Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoids becomes very difficult.
(ii) There is similarity in size of elements belonging to same group of second and third transition series.
Example: Zr and Hf are known as chemical twins due to their almost identical radii.
Question 60.
What is meant by ‘’disproportionation’? Give one example of disproportionation reaction in aqueous solutions. (Comptt. All India 2015)
Answer:
Disproportionation: When in a reaction, the oxidation of an element in a compound increases in one of the products and decreases in the other product, it is said to undergo disproportionation of oxidation state. In other words, the reaction in which an element undergoes self-oxidation and self-reduction simultaneously.
Example : In acidic solution Mn (VI) in
changes to Mn (VII) in the product 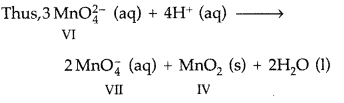
Question 61.
Give reasons:
(i) Mn shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4.
(ii) Transition metals show variable oxidation states.
(iii) Actinoids show irregularities in their electronic configurations. (Delhi 2016)
Answer:
(i) Because oxygen stabilizes the highest oxidation state (+7 of Mn) even more than fluorine i.e., +4 since oxygen has the ability to form multiple bonds with metal atoms.
(ii) The variability of oxidation state of transition elements is due to incompletely filled d-orbitals and presence of unpaired electrons.
(iii) This happens because the energy difference between 5f, 6d and 7s subshells of the actinoids is very small and hence electrons can be accomodated in any of them.
Question 62.
In the 3d series (Sc = 21 to Zn = 30) :
(i) Which element shows maximum number of oxidation states?
(ii) Which element shows only +3 oxidation state?
(iii) Which element has the lowest enthalpy of atomization? (Comptt. Delhi 2016)
Answer:
(i) Mn
(ii) Sc
(iii) Zn
Question 63.
Describe the preparation of potassium permangnate. How does the acidified permanganate solution react with oxalic acid? (Comptt. All India 2016)
Answer:
Pyrollusite ore is digested in KOH in the presence of oxygen
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3MnO42- + 4H+ → 2MnO4– + MnO2 + 2H2O
Reaction with oxalic acid in Acidic medium
5C2O42- + 2MnO4– + 16H+ → 2Mn+2 + 8H2O + 10CO2
Question 64.
Define lanthanoid contraction. Write its two consequences. (Comptt. All India 2016)
Answer:
Steady and regular decrease in atomic or ionic size with increasing atomic no. in Lanthanoid series.
Consequences :
- Due to lanthanoid contraction elements of 4d and 5d have similar size, hence occurs together.
- Basic character of trivalent hydroxide decreases.
Question 65.
A mixed oxide of iron and chromium is fused with sodium carbonate in free access of air to form a yellow coloured compound (A). On acidification the compound (A) forms an orange coloured compound (B), which is a strong oxidizing agent. Identify compound (A) and (B). Write chemical reactions involved. (Comptt. All India 2017)
Answer:
The mixed oxide of iron and chromium is chromite or chrome ion i.e. FeO.Cr2O3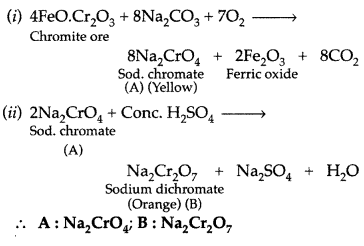
Question 66.
(a) Give reasons for the following:
(i) Compounds of transition elements are generally coloured.
(ii) MnO is basic while Mn2O7 is acidic.
(iii) Calculate the magnetic moment of a divalent ion in aqueous medium if its atomic number is 26. (Comptt. All India 2017)
Answer:
(a) (i) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d- orbitals and variable oxidation states. The colour of transition metal ions is due to d-d transition. When electrons jump from one orbital to another in their partially filled d-orbitals, another light is emitted due to which the compounds of transition elements seem to be coloured.
(ii) MnO is basic while Mn2O7 is acidic because the basic nature decreases as the oxidation state or number of oxygen atoms increases i.e. MnO (+4) and Mn2O7 (+7)
(b) Divalent ion with atomic number 26 is Fe2+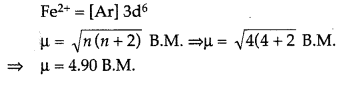
The d- and f-Block Elements Class 12 Important Questions Long Answer Type [LA]
Question 67.
(a) Complete the following chemical reaction equations :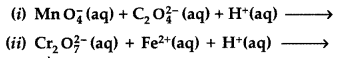
(b) Explain the following observations about the transition/inner transition elements :
(i) There is in general an increase in density of element from titanium (Z = 22) to copper (Z = 29).
(ii) There occurs much more frequent metal-metal bonding in compounds of heavy transition elements (3rd series).
(iii) The members in the actinoid series exhibit a larger number of oxidation states than the corresponding members in the lanthanoid series. (Delhi & All India 2009)
Answer:
(a)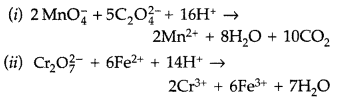
(b) (i) As we move along a transition series from left to right, the atomic radii decrease due to increase in nuclear charge. Hence, the atomic volume decreases. At the same time, atomic mass increases from Ti to Cu, therefore density increases.
(ii) It is due to their low ionization energies and variable oxidation state.
(iii) Because of very small energy gap between 5f, 6d and 7s subshells all their electrons can take part in bonding and shows variable oxidation states.
Or
Because 5f electrons are less burned than 4 ‘f’ electrons. Hence 5f electrons are also taking part in chemical bonding. Hence actinoids are showing large number of oxidation states.
Question 68.
(a) Complete the following chemical equations for reactions :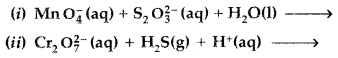
(b) Give an explanation for each of the following observations :
(i) The gradual decrease ‘n’ size (actinoid contraction) from element to element is greater among the actinoids than that among the lanthanoids (lanthanoid contraction).
(ii) The greatest number of oxidation states are exhibited by the members in the middle of a transition series.
(iii) With the same d-orbital configuration d4, Cr2+ion is a reducing agent but Mn3+ ion is an oxidising agent. (Delhi 2009)
Answer:
(a)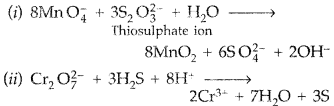
(b) (i) The actinoid contraction is greater than lanthanoid contraction due to poorer shielding of 5f electrons as they are extended in space beyond 6s and 6p orbitals whereas 4f orbitals are buried deep inside the atom.
(ii) Due to presence of more unpaired electrons and use of all 4s and 3d electrons in the middle of series.
(iii) Cr2+ has the configuration 3d4 which easily changes to d3 due to stable half filled t2g orbitals. Therefore Cr2+ is reducing agent. While Mn2+ has stable half filled d5 configuration. Hence Mn3+easily changes to Mn2+ and acts as oxidising agent.
Question 69.
(a) Complete the following chemical reaction equations :
(i) Fe2+(aq) +
(ii)
(b) Explain the following observations :
(i) Transition elements are known to form many interstitial compounds.
(ii) With the same d4 d-orbital configuration Cr2+ion is reducing while Mn3+ ion is oxidizing.
(iii) The enthalpies of atomization of the transition elements are quite high. (Delhi 2009)
Answer:
(a) Fe2+(aq) +
(ii)
(b) (i) The transition metals form a large number of interstitial compounds in which small atoms such as hydrogen, carbon, boron and nitrogen occupy the empty spaces in the crystal lattices of transition metals.
(ii) Cr2+ has the configuration 3d4 which easily changes to d3 due to stable half filled t2g orbitals. Therefore Cr2+ is reducing agent. While Mn2+ has stable half filled d5 configuration. Hence Mn3+easily changes to Mn2+ and acts as oxidising agent.
(iii) Enthalpy of atomization is the amount of heat required to break the metal lattice to get free atoms. As transition metals contain a large number of unpaired electrons, they have strong interatomic attractions (metallic bonds). Hence they have high enthalpies of atomization.
Question 70.
(a) Complete the following chemical reaction equations :
(i)
(ii) MnO2(s) + KOH(aq) + O2 →
(b) Explain the following observations :
(i) Transition metals form compounds which are usually coloured.
(ii) Transition metals exhibit variable oxidation states.
(iii) The actinoids exhibit a greater range of oxidation states than the lanthanoids. (Delhi 2009)
Answer:
(a)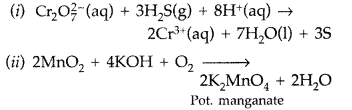
(b) (i) Colour is due to the presence of unpaired electrons in their d-subshells.
(ii) The variability of oxidation state of transition elements is due to incompletely filled d-orbitals. In transitional elements ns and (n – 1)d electrons have approximate equal energies hence in addition to ns electrons, (n – 1)d electrons are also taking part in chemical bonding.
(iii) Because of very small energy gap between 5f, 6d and 7s subshells all their electrons can take part in bonding and shows variable oxidation states.
Question 71.
(a) Complete the following chemical reaction equations :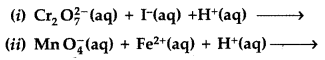
(b) Explain the following observations :
(i) In general the atomic radii of transition elements decrease with atomic number in a given series.
(ii) The E°M2+/M, for copper is positive (+ 0.34 V). It is the only metal in the first series of transition elements showing this type of behaviour.
(iii) The E° value for Mn3+ | Mn2+ couple is much more positive than for Cr3+ | Cr2+ or Fe3+ | Fe2+couple. (Delhi 2009)
Answer:
(a) (i)
(ii) 5Fe2+ +
(b) (i) Because of increase in effective nuclear charge and weak shielding effect of d-electrons, the atomic radii decreases.
(ii) The E°M2+/M for any metal is related to the sum of the enthalpy changes taking place in the following steps :
M(g) + ΔaH → M(g) (ΔaH = enthalpy of atomization)
M(g) + ΔiH → M2+(g) (ΔiH = ionization enthalpy)
M2+(g) + aq → M2+(aq) + ΔhydH (ΔhydH = hydration enthalpy)
Copper has high enthalpy of atomization (i.e. energy absorbed and low enthalpy of hydration (i.e. energy released). Hence E°M2+/M for copper is positive. The high energy required to transform Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(iii) The large positive E° value for Mn3+ | Mn2+shows that Mn2+ is much more stable than Mn3+due to stable half filled configuration (3d5). Therefore the 3rd ionization energy of Mn will be very high and Mn3+ is unstable and can be easily reduced to Mn2+. E° value for Fe3+ | Fe2+ is positive but small i.e. Fe3+ can also be reduced to Fe2+ but less easily. Thus Fe3+ is more stable than Mn3+.
Question 72.
(a) What is meant by the term lanthanoid contraction? What is it due to and what consequences does it have on the chemistry of elements following lanthanoids in the periodic table?
(b) Explain the following observations :
(i) Cu+ ion is unstable in aqueous solutions.
(ii) Although Co2+ ion appears to be stable, it is easily oxidised to Co3+ ion in the presence of a strong ligand.
(in) The E°Mn2+/Mn value for manganese is much more than expected from the trend for other elements in the series. (Delhi 2009)
Answer:
(a) Lanthanoid contraction : The overall decrease in atomic and ionic radii with increasing atomic number is known as lanthanoid contraction.
Cause: As we move along the lanthanoid series, the effective nuclear charge increases on addition of electrons and the electrons added in f-subshell causes imperfect shielding which is unable to counterbalance the effect of the increased nuclear charge. Hence the contraction in size occurs.
Consequences :
(i) Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoids becomes very difficult.
(ii) There is similarity in size of elements belonging to same group of second and third transition series.
Example: Zr and Hf are known as chemical twins due to their similar radii.
(b) (i) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but Δhyd (hydration enthalpy) for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows :
2Cu+ → Cu2+ + Cu
(ii) Co2+ ion is easily oxidised to Co3+ ion in presence of a strong ligand because of its higher crystal field energy which causes pairing of electrons to give inner orbital complexes (d2sp3). Co3+ can accomodate more no. of electrons from the ligand and offer better stability to the resulting complex. Hence Co2+ oxidises to Co3+.
(iii) The value of E° for Mn is more negative than expected from the general trend due to greater stability of half filled d-subshell (d5) in Mn2+.
Question 73.
(a) Complete the following chemical equations :
(i)
(ii) Cu2+ (aq) + I–(aq) →
(b) How would you account for the following :
(i) The oxidising power of oxoanions are in the order
(ii) The third ionization enthalpy of manganese (Z = 25) is exceptionally high.
(iii) Cr2+ is a stronger reducing agent than Fe2+. (All India 2010)
Answer:
(a) (i) latex]\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}[/latex] + 3H2S + 8H+ → 2Cr3+ + 7H2O + 3S
(ii) 2Cu2+ (aq) + 4I– (aq) → Cu2I2 + I2
(b) (i) The ions in which the central metal atom is present in the highest oxidation state will have the highest oxidising power. In
VO2+ < Cr2O72- < MnO4–
(ii) Because third electron has to be removed from stable half filled 3d-orbitals (Z = 25 has 3d5 4s2).
(iii) Cr2+ is a stronger reducing agent than Fe2+because E°Cr3+/Cr2+ is negative (-0.41 V)
whereas E°Fe3+/Fe2+ is positive (0.77 V). Thus Cr2+ is easily oxidised to Cr3+ but Fe2+ cannot be easily oxidized to Fe3+.
Question 74.
(a) Complete the following chemical equations :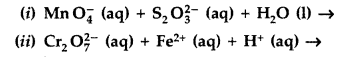
(b) Explain the following observations :
(i) La3+ (Z = 57) and Lu3+ (Z = 71) do not show any colour in solutions.
(ii) Among the divalent cations in the first series of transition elements, manganese exhibits the maximum paramagnetism.
(iii) Cu+ ion is not known in aqueous solutions. (All India 2010)
Answer: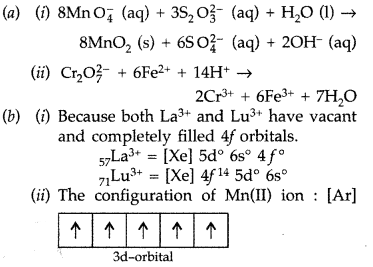
It has maximum five unpaired electrons, so maximum paramagnetism
(iii) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but Δhyd (hydration enthalpy) for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows :
2Cu+ → Cu2+ + Cu
Question 75.
(a) Describe the preparation of potassium dichromate from chromite ore. What is the effect of change of pH on dichromate ion?
(b) How is the variability in oxidation states of transition elements different from that of non-transition elements? Illustrate with examples. (Comptt. All India 2012)
Answer:
(a) Potassium dichromate is prepared from chromate by reacting chromite ore with Na2CO3
4 FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3+ 8CO2 .
The yellow solution of sodium chromate is filtered off and acidified with H2SO4 to give orange sodium dichromate
2Na2CrO4 + 2H+ → Na2Cr2O7 + H2O + 2Na
Sodium dichromate is then treated with KCl to give potassium dichromate as orange crystals.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
The chromates and dichromates are interconvertible in aqueous solution depending upon pH of the solution.
2CrO4-2 + 2H+ → Cr2O7-2 + H2O
Cr2O7-2 + 2OH–→ 2CrO4-2 + H2O
(b) In transition elements, the successive oxidation state differs by unity, e.g, Mn shows all the oxidation states from +2 to +7. On the other hand, non-transition metals exhibit variable oxidation states which differ by two units, e.g. Pb(II), Pb(IV), Sn(II), Sn(IV) etc.
Question 76.
(a) Describe the preparation of potassium permanganate from pyrolusite ore. What happens when acidified potassium permanganate solution reacts with ferrous sulphate solution? Write balanced chemical equations.
(b) Account for the following :
(i) Mn2+compounds are more stable than Fe2+compounds towards oxidation to their +3 state.
(ii) Cr2+ is reducing and Mn3+ oxidizing when both have d4 configuration. (Comptt. All India 2012)
Answer:
(a) KMnO4 is prepared by fusion of MnO2 with an alkali metal hydoxide and an oxidising agent like KNO3. This produces the dark green K2MnO4which disproportionates in a neutral or acidic solution to give KMnO4
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3MnO4-2 + 4H+ → 2MnO4– + MnO2 + 2H2O
Fe+2(green) is converted to Fe+3(yellow)
5Fe+2 + MnO4– + 8H+ → Mn+2+ 4H2O + 5Fe+3
(b) (i) The electronic configuration of Mn+2 is [Ar] 3d5 i.e. all the five d-orbitals are singly occupied. Thus, this configuration is stable and resists further oxidation i.e. loss of further electrons requires high energy. On the other hand, the electronic configuration of Fe+2 is [Ar]3d6. Hence it loses one electron and achieves the stable configuration i.e. oxidation of Fe+2 to Fe+3 is easily achieved.(ii) Cr+2 is reducing in nature as its configuration changes from d4 to d3 which is a stable configuration having half filled t2g orbitals. On the other hand, Mn+3 is oxidising in nature as the configuration changes from d4 to d5 (a stable configuration having half filled t2g and eg orbitals).
Question 77.
(a) Give reasons for the following :
(i) Mn3+ is a good oxidising agent.
(ii) E°M2+/M values are not regular for first row transition metals (3d series).
(iii) Although ‘F is more electronegative than ‘O’, the highest Mn fluoride is MnF4, whereas the highest oxide is Mn2O7.
(b) Complete the following equations : (All India 2013)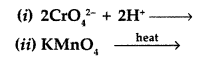
Answer:
(a) (i) Mn3+ has electronic configuration 3d44s0.
On reduction it gains one electron to become 3d54s0 which is half filled stable configuration. Hence it is a good oxidising agent.
(ii) E°M2+/M values are not regular for first row transition metals due to abnormalities and irregularities in their ionization enthalpies (IE1 + IE1) and sublimation enthalpies.
(iii) Because oxygen stabilizes the highest oxidation state even more than fluorine and has ability to form multiple bonds with metal atoms.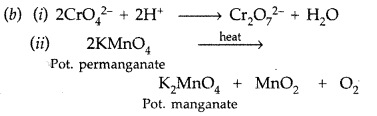
Question 78.
(a) Why do transition elements show variable oxidation states?
(i) Name the element showing maximum number of oxidation states among the first series of transition metals from Se (Z = 21) to Zn (Z = 30).
(ii) Name the element which shows only +3 oxidation state.
(b) What is lanthanoid contraction? Name an important alloy which contains some of the lanthanoid metals. (All India 2013)
Answer:
(a) Because the energy difference between (n-1) d-orbitals and ns-orbitals is very less. Since there is very little energy difference between these orbitals, both energy levels can be used for bond formation. Thus transition elements exhibit variable oxidation states.
(i) It is Manganese (Mn) which shows oxidation states from +2 to +7
(ii) Scandium (Sc) shows only +3 oxidation state.
(b)Lanthanoid contraction : The overall decrease in atomic and ionic radii with increasing atomic number is known as lanthanoid contraction. In going from La+3 to Lu+3 in lanthanoid series, the size of ion decreases. This decrease in size in the lanthanoid series is known as lanthanoid contraction. The lanthanoid contraction arises due to imperfect shielding of one 4f electron by another present in the same subshell.
Consequences :
(i) Similarity in properties : Due to lanthanoid contraction, the size of elements which follow (Hf – Hg) are almost similar to the size of the elements , of previous row (Zr – Cd) and hence these are difficult to separate. Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoid becomes very difficult.
(ii) Basicity difference : Due to lanthanoid contraction, the size decreases from La+3 to Lu+3. Thus covalent character increases. Hence basic character of hydroxides also decreases i.e. why La(OH)3 is most basic while Lu(OH)3 is least basic.
Important alloy: Mischmetal alloy which contains 95% lanthanoids and 5% Fe.
Question 79.
Give reasons :
(i) Zirconium (Z = 40) and Hafnium (Z = 72) have almost similar atomic radii.
(ii) d-block elements exhibit more oxidation states than f-block elements.
(iii) The enthalpies of atomization of the transition metals are high.
(iv) The variation in oxidation states of transition metals is of different type from that of the non-transition metals.
(v) Orange solution of potassium dichromate
turns yellow on adding sodium hydroxide to it. (Comptt. All India 2013)
Answer:
(i) Zirconium and Hafnium have almost similar atomic radii due to the effect of lanthanoid contraction.
(ii) d-block elements exhibit more oxidation states due to small energy gap between ns and (n – 1)d subshell while f – block elements show less oxidation state due to large energy gap between ns and (n -2)f subshell.
(iii) Enthalpy of atomization is the amount of heat required to break the metal lattice to get free atoms. As transition metals contain a large number of impaired electrons, they have strong interatomic attractions (metallic bonds). Hence they have high enthalpies of atomization.
(iv) In transition elements, the successive oxidation state differs by unity, e.g, Mn shows all the oxidation states from +2 to +7. On the other hand non-transition metals exhibit variable oxidation states which differ by two units, e.g. Pb(II), Pb(IV), Sn(II), Sn(IV) etc.
(v) The orange coloured potassium dichromate solution when treated with basic NaOH solution, is converted to chromate which gets a faint colour like yellow.
The reaction is given as:
K2Cr2O7 + 2NaOH → K2CrO4 + Na2CrO4 + H2O.
This formation of chromate (CrO4–) ion converts the colour of solution to yellow.
Question 80.
(a) Describe the preparation of potassium permanganate from pyrolusite ore. Write balanced chemical equation for one reaction to show the oxidizing nature of potassium permanganate.
(b) What is lanthanoid contraction and what is it due to? Write two consequences of lanthanoid contraction. (Comptt. All India 2013)
Answer:
(a) Preparation of KMnO4 from pyrolusite ore occurs in two steps :
(i) Conversion of Mn02 into potassium manganate :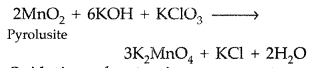
(ii) Oxidation of potassium manganate to potassium permanganate : The obtained potassium manganate bubbled with carbon dioxide or chlorine or ozonised oxygen.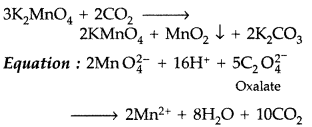
(b) Lanthanoid contraction : The overall decrease in atomic and ionic radii with increasing atomic number is known as lanthanoid contraction. In going from La+3 to Lu+3 in lanthanoid series, the size of ion decreases. This decrease in size in the lanthanoid series is known as lanthanoid contraction. The lanthanoid contraction arises due to imperfect shielding of one 4f electron by another present in the same subshell.
Consequences :
(i) Similarity in properties: Due to lanthanoid contraction, the size of elements which follow (Hf – Hg) are almost similar to the size of the elements , of previous row (Zr – Cd) and hence these are difficult to separate. Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoid becomes very difficult.
(ii) Basicity difference: Due to lanthanoid contraction, the size decreases from La+3 to Lu+3. Thus covalent character increases. Hence basic character of hydroxides also decreases i.e. why La(OH)3 is most basic while Lu(OH)3 is least basic.
Question 81.
(a) How do you prepare :
(i) K2MnO4 from MnO2?
(ii) Na2Cr2O7 from Na2CrO4?
(b) Account for the following :
(i) Mn2+ is more stable than Fe2+ towards oxidation to +3 state.
(ii) The enthalpy of atomization is lowest for Zn in 3d series of the transition elements.
(iii) Actinoid elements show wide range of oxidation states. (Delhi 2014)
Answer:
(a) (i) K2MnO4 from MnO2 (Pyrolusite) :
Finely powdered pyrolusite is fused with KOH in the presence of air to give green coloured potassium manganate.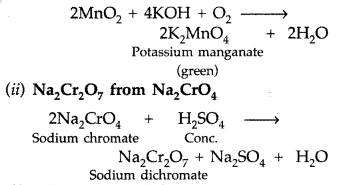
(b) (i) The 3d orbital in Mn2+ is half-filled and is more stable compared to Fe2+ has 6 electrons in the 3d orbital. Mn2+ prefer to lose an electron or get oxidised whereas Fe2+ will readily loose one electron or get oxidised. Therefore, Mn2+ is much more resistant than Fe2+ towards oxidation.
(ii) As there are no unpaired electrons in zinc, it is soft and has low melting point and low enthalpy of atomization.
(iii) Because of very small energy gap between 5f, 6d and 7s subshells all their electrons can take part in bonding and shows variable oxidation states.
Question 82.
(i) Name the element of 3d transition series which shows maximum number of oxidation states. Why does it show so?
(ii) Which transition metal of 3d series has positive E0(M2+/M) value and why?
(iii) Out of Cr3+ and Mn3+, which is a stronger oxidizing agent and why?
(iv) Name a member of the lanthanoid series which is well known to exhibit +2 oxidation state.
(v) Complete the following equation :
MnO4– + 8H+ + 5e– → (Delhi 2014)
Answer:
(i) Mn has the maximum number of unpaired electrons present in the d-subshell (5 electrons). Hence, Mn exhibits the largest number of oxidation states, ranging from +2 to +7.
(ii) Copper has positive E0(Cu2+/Cu) value because of its high enthalpy of atomization and low enthalpy of hydration. The high energy required to oxidise Cu to Cu2+ is not balanced by its hydration energy.
(iii) Cr2+ has the configuration 3d4 which easily changes to d3 due to stable half filled t2g orbitals. Therefore Cr2+ is reducing agent, it gets oxidized to Cr3+. While Mn2+ has stable half filled d5configuration. Hence Mn3+ easily changes to Mn2+and acts as oxidising agent.
(iv) Eutropium is well known to exhibit +2 oxidation state.
(v) MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
Question 83.
(a) Complete the following equations :
(i) Cr2O72- + 2OH– →
(ii) MnO4– + 4H+ + 3e– →
(b) Account for the following :
(i) Zn is not considered as a transition element.
(ii) Transition metals form a large number of complexes.
(iii) The E0 value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+ couple. (All India 2014)
Answer:
(a) (i) Cr2O72- + 2OH– → 2CrO42- + H2O
(ii) MnO4– + 2H2O + 3e– → MnO2 + 4OH–
(b) (i) Zinc in its common oxidation state of +2 has completely filled d-orbitals. Hence considered as non-transition elements.
(ii) Because of smaller size of their ions, high ionic charge and availability of vacant d-orbitals, transition metals from a large number of complexes.
(iii) The large positive E° value for Mn3+ | Mn2+shows that Mn2+ is much more stable than Mn3+due to stable half filled configuration (3d5). Therefore the 3rd ionization energy of Mn will be very high and Mn3+ is unstable and can be easily reduced to Mn2+. E° value for Fe3+ | Fe2+ is positive but small i.e. Fe3+ can also be reduced to Fe2+ but less easily. Thus Fe3+ is more stable than Mn3+.
Question 84.
(i) With reference to structural variability and chemical reactivity, write the difference between lanthanoids and actinoids.
(ii) Name a member of the lanthanoid series which is well known to exhibit +4 oxidation state.
(iii) Complete the following equation :
MnO4– + 8H+ + 5e– →
(iv) Out of Mn3+ and Cr3+, which is more paramagnetic and why? (Atomic nos. : Mn = 25, Cr = 24)(All India 2014)
Answer:
(i) Difference between lanthanoids and actinoids :
- The general electronic configuration of lanthanoids is [Xe]54 4f1-14 5d0-1 6s2 whereas that of actinoids is [Rn86 5f1-14 6d0-1 7s2. Thus lanthanoids belong to 4/ series whereas actinoids belong to 5/ series.
- Actinoids are radioactive while lanthanoids are not radioactive.
- The ionisation enthalpies of the early actinoids are lower than those of the early lanthanoids. Actinoids show the oxidation state from +3 (most common) to +7, while lanthanoids show the oxidation state from +3 up to + 7.
- The first few members of the lanthanoids series are quite reactive. Therefore they combine with H2 on gentle heating while the actinoids are highly reactive especially in the finely divided state, therefore they combine with most of the non-metals at moderate temperature.
(ii) Cerium
(iii) MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
(iv) Mn3+ is more paramagnetic due to presence of 4 unpaired electrons than Cr3+ having 3 unpaired electrons.
Question 85.
(a) Account for the following:
(i) Mn shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4.
(ii) Cr2+ is a strong reducing agent.
(iii) Cu2+ salts are coloured while Zn2+ salts are white.
(b) Complete the following equations: (All India 2016)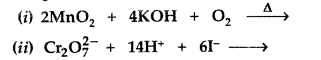
Answer:
(a) (i) Because oxygen stabilizes the highest oxidation state (+7 of Mn) even more than fluorine i.e., +4 since oxygen has the ability to form multiple bonds with metal atoms.
(ii) Cr2+ exists in the d4 system and is easily oxidized to Cr3+ by loosing one electron which has the stable d3/t2g orbital configuration. So, Cr2+ is a strong reducing agent.
(iii) Cu2+ has the configuration 3d9 with one unpaired electron which gets excited in the visible region to impart its colour while Zn2+ has 3d10configuration without any unpaired electron so no d – d transition possible and hence colourless.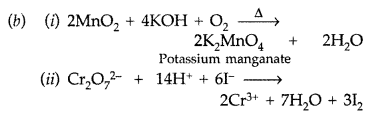
Question 86.
The elements of 3d transition series are given as:
Se Ti V Cr Mn Fe Co Ni Cu Zn
Answer the following:
(i) Write the element which shows maximum number of oxidation states. Give reason.
(ii) Which element has the highest m.p.?
(iii) Which element shows only +3 oxidation state?
(iv) Which element is a strong oxidizing agent in +3 oxidation state and why? (All India)
Answer:
(i) Mn shows, maximum number of oxidation states upto +7. It has the maximum number of unpaired electrons.
(ii) Cr has the highest melting point.
(iii) Sc shows only +3 oxidation state.
(iv) Mn is a strong oxidizing agent in +3 oxidation state because after reduction it attains +2 oxidation state in which it has the most stable half-filled (d5) configuration.
Question 87.
(a) Account for the following:
(i) Transition metals form large number of complex compounds.
(ii) The lowest oxide of transition metal is basic whereas the highest oxide is amphoteric or acidic.
(iii) E° value for the Mn3+/Mn2+ couple is highly positive (+1.57 V) as compared to Cr3+/Cr2+.
(b) Write one similarity and one difference between the chemistry of lanthanoid and actinoid elements. (Delhi 2017)
Answer:
(a) (i) Transition metals form large number of complexes because:
- small size of metal ion;
- high ionic charge; and
- availability of empty d-orbitals.
(ii) The lowest oxide of transition metal is basic because of low oxidation state some of valence electrons are not involved in bonding and acts as base by donating electron. However in higher oxides due to high oxidation state, it cannot donate electrons but can accept electrons due to high effective nuclear charge. Hence, they are acidic in nature.
(iii) Mn2+ exists in half-filled d5 state which is very stable while Mn3+ is d4 which is not so stable. Mn3+ can be easily reduced to Mn2+. Conversion from d4 to d5 will be quick and have negative ΔG value. Hence, because of the stability factor the E° value is high for this process. While Cr3+ is d3 is half-filled (t2g3) is stable in nature and Cr2+ is d4, has one extra electron which it would like to donate to attain the stable half-filled (t2g3) configuration. Hence for the process Cr3+ to Cr2+, the value of E° is less.
(b) Similarity: Both lanthanoids and actinoids show contraction in size and irregularity in their electronic configuration.
Difference: Actinoids show wide rage of oxidation states but lanthanoids do not.
Question 88.
(a) (i) How is the variability in oxidation
states of transition metals different from that of the p-block elements?
(ii) Out of Cu+ and Cu2+, which ion is unstable in aqueous solution and why?
(iii) Orange colour of Cr2O72- ion changes to yellow when treated with an alkali. Why?
(b) Chemistry of actinoids is complicated as compared to lanthanoids. Give two reasons. (Delhi 2017)
Answer:
(a) (i) In transition elements, the oxidation
states can vary from +1 to highest oxidation state by removing all its valence electrons and the oxidation states differ by 1, e.g., Fe2+ and Fe3+while in p-block elements, the oxidation states differ by 2, e.g., +2 and +4 or +3 and +5, etc.
(ii) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but ΔhydH for Cu2+(aq) is much more negative that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows:
2Cu+ → Cu2+ + Cu
(iii) The orange coloured potassium dichromate solution when treated with basic NaOH solution, is converted to chromate which gets a faint colour like yellow.
The reaction is given as:
K2Cr2O7 + 2NaOH → K2CrO4 + Na2CrO4 + H2O.
This formation of chromate (CrO4–) ion converts the colour of solution to yellow.
(b)
- Lanthanoids show limited oxidation states, i.e., +2, +3, +4 out of which +3 is most common which is due to large energy gap between 4f and 5d subshells while actinoids show large number of oxidation states due to small energy gap between 5f, 6d and 7s subshells.
- Actinoid contraction is greater than lanthanoid contraction due to poor shielding of 5f electrons.
- Actinoids are radioactive in nature.
Question 89.
(a) Account for the following:
(i) Transition metals show variable oxidation states.
(ii) Zn, Cd and Hg are soft metals.
(iii) E° value for the Mn3+/Mn2+ couple is highly positive (+1.57 V) as compared to Cr3+/Cr2+.
(b) Write one similarity and one difference between the chemistry of lanthanoid and actinoid elements. (All India 2017)
Answer:
(a) (i) The variability of oxidation state of transition elements is due to incompletely filled d-orbitals. In transitional elements ns, and (n – 1) d electrons have a approximate equal energies hence in addition to ns electrons, (n – 1) d electrons are also taking part in chemical bonding.
(ii) Zn, Cd and Hg are soft metals because they do not exhibit covalency due to completely filled d-orbitals. Absence of unpaired d electrons causes weak metallic bonding.
(iii) Mn2+ exists in half-filled d5 state which is very stable while Mn3+ is d4 which is not so stable. Mn3+ can be easily reduced to Mn2+. Conversion from d4 to d5 will be quick and have negative ΔG value. Hence, because of the stability factor the E° value is high for this process. While Cr3+ is d3 is half-filled (t2g3) is stable in nature and Cr2+ is d4, has one extra electron which it would like to donate to attain the stable half-filled (t2g3) configuration. Hence for the process Cr3+ to Cr2+, the value of E° is less.
(b) Similarity: Both lanthanoids and actinoids show contraction in size and irregularity in their electronic configuration.
Difference: Actinoids show wide rage of oxidation states but lanthanoids do not.
Question 90.
(a) Following are the transition metal ions of 3d series:
Ti4+, V2+, Mn3+, Cr3+
(Atomic numbers: Ti = 22, V= 23, Mn = 25, Cr = 24)
Answer the following:
(i) Which ion is most stable in an aqueous solution and why?
(ii) Which ion is a strong oxidising agent and why?
(iii) Which ion is colourless and why?
(b) Complete the following equations: (All India 2017)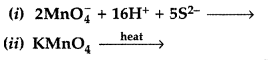
Answer:
(a) (i) Cr3+ is most stable because of its small size and t32g configuration.
(ii) Mn3+ is a strong oxidising agent because after gaining one electron it is converted into Mn2+which has stable d5 configuration.
(iii) Ti4+ is colourless due to d° configuration, i.e., no unpaired electrons.
(b) (i) 2MnO4– + 16H+ + 5S2- → 2Mn2+ + 8H2O + 5S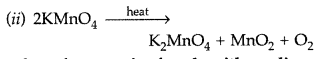
Question 91.
When chromite ore is fused with sodium carbonate in free excess of air and the product is dissolved in water, a yellow solution of compound (A) is obtained. On acidifying the yellow solution with sulphuric acid, compound (B) is crystallised out. When compound (B) is treated with KC1, orange crystals of compound (C) crystallise out. Identify (A), (B) and (C) and write the reactions involved. (Comptt. Delhi 2017)
Answer:
Fusion of chromite ore with sodium carbonate :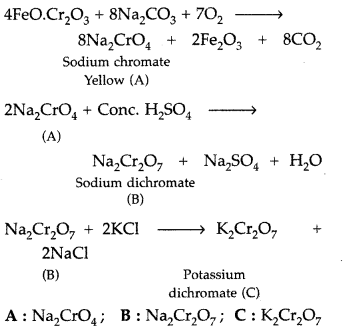
Question 92.
(a) (i) Which transition element in 3d series has positive E0M2+/M and why?
(ii) Name a member of lanthanoid series which is well know to exhibit +4 oxidation state and why?
(b) Account for the following :
(i) The highest oxidation state is exhibited in oxoanions of transition metals.
(ii) HCl is not used to acidify KMnO4 solution.
(iii) Transition metals have high enthalpy of atomisation. (Comptt. Delhi 2017)
Answer:
(a) (i) Copper has positive E0M2+/M value because the sum of enthalpies of sublimation and ionization is not balanced by hydration enthalpy.
(ii) Cerium shows +4 oxidation state because it acquires stable empty orbital configuration and therefore Ce+4 is also used as a good analytical reagent and good oxidising agent.
(b) (i) The highest oxidation state shown in oxoanions of transition metals is
(ii) HCl is not used to the acidify KMnO4 solution because KMnO4 is a very strong oxidizing agent and it can oxidize HCl to liberate chlorine gas.
(iii) In transition elements, there are large number of unpaired electrons in their atoms, thus they have a stronger inter atomic interaction and thereby stronger bonding between the atoms. Due to this they have high enthalpies of atomization.
No comments:
Post a Comment