Important Questions
Question 1.
Why is Bi(v) a stronger oxidant than Sb(v)? (Delhi 2009)
Answer:
The stability of +5 oxidation state decreases and that of +3 state increases due to inert pair effect down the group therefore Bi(v) accepts two electrons and gets reduced to Bi (v).
Bi5+ + 2e– → Bi3+
Question 2.
Which is a stronger oxidizing agent Bi(v) or Sb(v)? (Delhi 2009)
Answer:
Bi(v) is stronger oxidizing agent due to inert pair effect.
Question 3.
Why is red phosphorus less reactive than white phosphorus? (All India 2009)
Answer:
Because white phosphorus has angular strain in its P4 molecules where the angle is only 60°.
Question 4.
Why does NO2 dimerise? (Delhi 2010)
Answer:
NO2 contains 7 + 2 × 8 i.e. 23 odd electrons. In the valence shell N has seven electrons and hence less stable. To acquire stability it dimerizes to form N2O4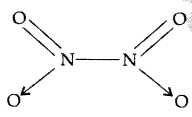
Question 5.
What is the oxidation number of phosphorus in H3PO2 molecule? (Delhi 2010)
Answer:
H3PO2
3 + x- 4 = 0 or x – 1 = 0 ∴ x = + 1
Thus oxidation number of P in H3PO2 = +1.
Question 6.
Draw the structure of 03 molecule. (Delhi 2010)
Answer:
Structure of Ozone (Os) : Angular structure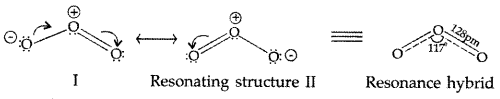
Question 7.
Fluorine does not exhibit any positive oxidation state. Why? (All India 2010)
Answer:
Since fluorine is the most electronegative element and does not have d-orbitals in its valence shell, therefore, it cannot expand its octet and hence does not show positive oxidation state (O.S.) while other halogens have d-orbitals and therefore exhibit many oxidation states.
Question 8.
Give the IUPAC name of the following compound : (All India 2010)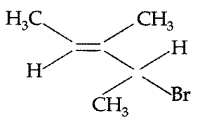
Answer:
IUPAC name : 2-Bromo-3-methylpent-3-ene
Question 9.
Nitrogen is relatively inert as compared to phosphorus. Why? (All India 2010)
Answer:
Because P -P single bond is much weaker than N = N triple bond and the bond length of nitrogen is small and bond dissociation energy is very large which makes it inert and urtreactive and thus phosphorus becomes more reactive.
Question 10.
Draw the structure of XeF2 molecule. (Delhi 2011)
Answer:
XeF2: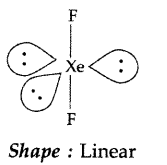
Question 11.
Draw the structure of XeF4 molecule. (Delhi 2011)
Answer:
XeF4 : sp3d2 hybridization
Shape → Square planar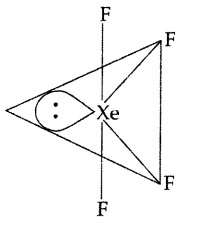
Question 12.
Draw the structure of BrF3 molecule. (Delhi 2011)
Answer:
BrF3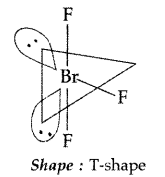
Question 13.
Which one of
Answer:
Question 14.
Of PH3 and H2S which is more acidic and why? (Delhi 2012)
Answer:
H2S, because of higher electronegativity of sulphur.
Question 15.
Which is a stronger reducing agent, SbH3 or BiH3, and why? (All India 2012)
Answer:
BiH3 : Because it is stronger reducing agent as its tendency to liberate H is maximum.
Question 16.
What is the basicity of H3PO2 acid and why? (All India 2012)
Answer:
H3PO2 has one replaceable H atom so it is monobasic.
Question 17.
Though nitrogen exhibits +5 oxidation state, it does not form pentahalide. Why? (Comptt. Delhi 2012)
Answer:
Due to non-availability of d-orbitals in its valence electronic configuration nitrogen does not form pentahalide.
Question 18.
Noble gases have low boiling points. Why? (Comptt. Delhi 2012)
Answer:
Noble gases being monoatomic have no interatomic forces except weak dispersion force and therefore, they are liquified at very low temperature. Hence, they have low boiling point.
Question 19.
Write a reaction to show the reducing behaviour of H3PO2. (Comptt. Delhi 2012)
Answer:
H3PO2 reduces AgNO3 to metallic Ag :
4AgNO3 + 2H2O + H3PO2 → 4Ag + 4HNO3 + H3PO4
Question 20.
Why does PCl3 fume in moisture? (Comptt. Delhi 2012)
Answer:
PCl3 hydrolyses in moisture giving fumes of HCl.
PCl3 + 3H2O → H3PO3 + 3HCl
Question 21.
Why does NO2 dimerise ? (Comptt. All India 2012)
Answer:
NO3 contains odd number of valence electrons. It behaves as a typical odd molecule. On dimerisation, it is converted to stable N2O4molecule with even number of electrons.
Question 22.
Why is ICl more reactive than I2? (Comptt. All India 2012)
Answer:
ICl is more reactive than I2 because I-Cl bond is weaker than I-I bond of I2.
Question 23.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements? (Comptt. All India 2012)
Answer:
Reducing nature depends upon the stability of M- H bond. As the stability of the bond decreases from N to Bi hydrides, BiH3 is the strongest reducing agent.
Question 24.
What is the covalency of nitrogen in N2O5? (Delhi 2013)
Answer:
The covalency of nitrogen in N2O5 is 4 because each nitrogen atom has four shared pairs of electrons.
Question 25.
What inspired N.Bartlett for carrying out reaction between Xe and PtF6? (Delhi 2013)
Answer:
Since PtF6 oxidises Osub>2 to O2+, Bartlett thought that PtF6 should also oxidise Xe to Xe+because the ionization enthalpies of O2 (1175 kj mol-1) and Xe (1170 kj mol-1) are quite close.
Question 26.
What happens when ethyl chloride is treated with aqueous KOH? (Delhi 2013)
Answer: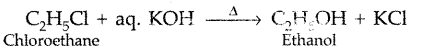
Question 27.
Name two poisonous gases which can be prepared from chlorine gas. (All India 2013)
Answer:
(i) Phosgene gas (COCl2) and (ii) Chloropicrin or tear gas (CCl3NO2).
Question 28.
Which aerosol depletes ozone layer? (All India 2013)
Answer:
Aerosols like foams, sprays etc. contain freons which are responsible for depletion of ozone layer.
Question 29.
What is the basicity of H3PO5 and why? (All India 2013)
Answer:
The basicity of H3PO5 is 2 because it contains only two ionizable H-atoms which are present as OH groups.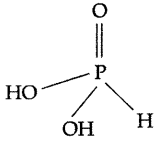
Questions 30.
Why does PCl3 fume in moisture? (Comptt. Delhi 2013)
Answer:
Phosphorus trichloride reacts readily with water giving phosphorus acid and hydrochloric acid. Reaction is very quick and exothermic![]()
Question 31.
Draw the structure of H3PO2 molecule. (Comptt. Delhi 2013)
Answer:
Cl2 + H2O → [HCl + HOCl] → 2HCl + [O]
Question 32.
Fluorine exhibits only -1 oxidation state whereas other halogens exhibit +1, +3, +5 and +7 oxidation states also. Why is it so? (Comptt. Delhi 2013)
Answer:
It is because fluorine is the most electronegative element and it does not have d-orbitals.
Question 33.
Though nitrogen exhibits +5 oxidation state, it does not form pentahalide. Why? (Comptt. All India 2013)
Answer:
Due to absence of d-subshell in N atom.
Question 34.
Bond enthalpy of fluorine is lower than that of chlorine. Why? (Comptt. All India 2013)
Answer:
Because F2 is very small and its interelectronic repulsions between the lone pairs of electrons are very large.
Question 35.
Write the structural formula of PCl5(s). (Comptt. All India 2013)
Answer:
PCl5(s)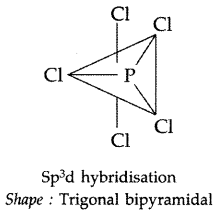
Question 36.
HF is a weaker acid than HCI. Why? (Comptt. All India 2013)
Answer:
Because of higher bond dissociation energy and strong H-bonding in HF.
Question 37.
Draw the structure of XeF2 molecule. (Comptt. All India 2013)
Answer:
XeF2 :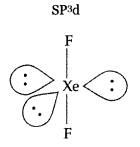
Question 38.
What is the basicity of H3PO3? (All India 2014)
Answer:
Basicity of H3PO3 = 2
Because basicity is the number of replaceable H+ions in an acid and H3PO3 is a Dibasic acid.
Question 39.
Why does NOz dimerise? (All India 2014)
Answer:
NO2 contains 7 + 2 × 8 i.e. 23 odd electrons. In the valence shell N has seven electrons and hence less stable. To acquire stability it dimerizes to form
N2O4.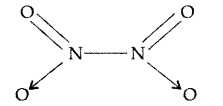
Question 40.
Why does NH3 act as a Lewis base? (All India 2014)
Answer:
Due to presence of lone pair on nitrogen NH3 acts as .a Lewis base.
Question 41.
Why is F2 a stronger oxidising agent than Cl2? (Comptt. All India 2014)
Answer:
Due to low bond dissociation enthalpy and high electronegativity of Fluorine, it has strong tendency to accept electrons and thus get reduced.
F + e– → F–
Therefore F2 acts as strong oxidising agent, while Cl2 is weak oxidising agent due to low electronegativity.
Question 42.
What is the basicity of H3PO4? (Delhi 2015)
Answer:
Since there are 3 OH groups present in II H3PO4, its basicity is 3.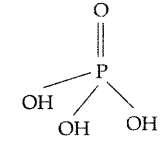
Question 43.
Write the formulae of any two oxoacids of sulphur. (All India 2015)
Answer:
H2SO3 and H2SO4
Question 44.
On adding NaOH to ammonium sulphate, a colourless gas with pungent odour is evolved which forms a blue coloured complex with Cu2+ion. Identify the gas. (Delhi 2016)
Answer:
The gas with a pungent odour is Ammonia (NH3) and the blue coloured complex is Tetra-ammine copper (II) sulphate monohydrate.
Question 45.
Pb(NO3)2 on heating gives a brown gas which undergoes dimerization on cooling. Identify the gas. (All India 2016)
Answer:
The brown gas is nitrogen dioxide (NO2) which can dimerize to N2O4
2Pb(NO3)2
Question 46.
Write the formula of the compound of phosphorus which is obtained when cone. HNO3 oxidises P4. (All India 2017)
Answer: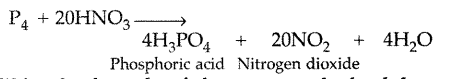
Question 47.
Write the formula of the compound of sulphur which is obtained when cone. HNO3 oxidises S8(All India 2017)
Answer:
S8 + 48HNO3 → 8H3SO4 + 48NO3 + 16H3O
Question 48.
Write the formula of the compound of iodine which is obtained when cone. HNO3 oxidises I2 . (All India 2017)
Answer:
I2 – 10HNO3 → 2HIO3 + 10NO2 + 4H3 O
Question 49.
Draw the structures of the following molecules :
(i) XeF4(it) BrF3 (Delhi 2009)
Answer:
(i) XeF4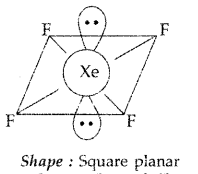
(ii) BrF3
Question 50.
Complete the following chemical reaction equations : (Delhi 2009)
(i) P4 (s) + NaOH (aq) + H2O (1) →
(ii) I– (aq) + H2O (1) + O3 (g) →
Answer: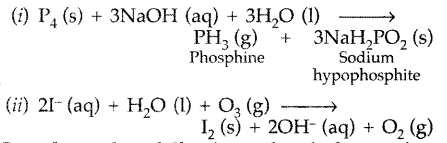
Question 51.
Complete the following chemical reaction equations : (All India 2009)
(i) XeF2 (s) + H2O (l) →
(ii) PH3 + HgCl2 →
Answer: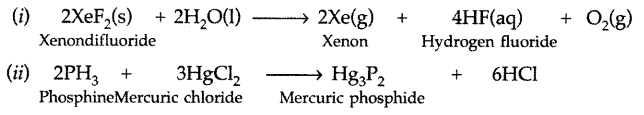
Question 52.
Draw the structures of white phosphorus and red phosphorus, phosphorus is more reactive and why? Which one of these two types of (Delhi 2010)
Answer: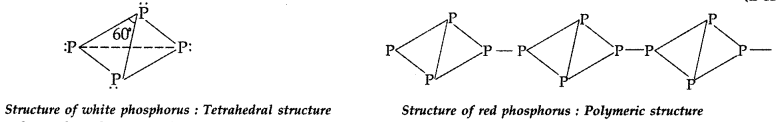
White phosphorus is more reactive due to its discrete tetrahedral structure and angular strain.
Question 53.
Draw the structural formulae of molecules of following compounds :
(i) BrF3 and (ii) XeF4 (Delhi 2010)
Answer:
(i) BrF3
(ii) XeF4
Question 54.
Complete the following chemical reaction equations : (All India 2010)
(i) I2 + HNO3(Conc.) → (ii) HgCl2 + PH3 →
Answer: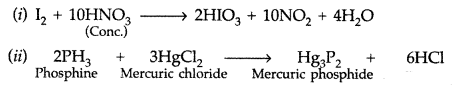
Question 55.
Draw the structural formulae of the following compounds :
(i) H4P2O5
(ii) XeF4 (All India 2010)
Answer:
Question 56.
Complete the following chemical reaction equations : (All India 2010)![]()
Answer: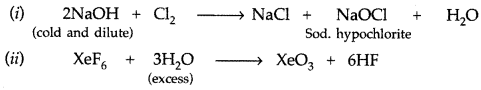
Question 57.
State reasons for each of the following :
(i) The N-O bond in
(ii) SF6 is kinetically an inert substance. (Delhi 2011)
Answer:
(i) The resonating structure of
Answer: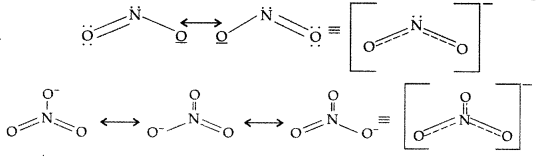
(ii) Because SF6 is showing steric hindrance due to 6 (six) fluorine atoms which make it unable to react further with any other atom.
Question 58.
State reasons for each of the following :
(i) All the P-Cl bonds in PCl5 molecule are not equivalent.
(ii) Sulphur has greater tendency for catenation than oxygen. (Delhi 2011)
Answer:
(i) The PCl5 molecule has sp3d hybridization and trigonal bipyramidal geometry. Therefore it has 3 equatorial P – Cl bonds and two axial P-Cl bonds. Since two axial P-Cl bonds are repelled by 3 bond pairs while 3 equatorial bonds are repelled by two bond pairs, so axial bonds are longer than equatorial bonds.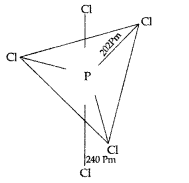
(ii) The greater catenation tendency of sulphur is due to two reasons :
(a) The lone pair of electrons feels more repulsion in 0-0 bond than S-S bond due to its small size and thus S-S forms strong bond.
(b) As the size of atom increases down the group from O – PO, the strength of bond increases and therefore catenation tendency also increases.
Question 59.
Explain the following giving an appropriate reason in each case. (Delhi 2012)
(i) O2 and F2 both stabilize higher oxidation states of metals but O2 exceeds F2 in doing so.
(ii) Structures of Xenon fluorides cannot be explained by Valence Bond approach.
Answer:
(i) This is due to the ability of oxygen to form multiple bonds to metals.
(ii) This is because the energy required for the promotion of electrons in Xenon is very high. Energy factor does not favour VB approach.
Question 60.
Explain the following facts giving appropriate reason in each case :
(i) NF3 is an exothermic compound whereas NCl3is not.
(ii) All the bonds in SF4 are not equivalent. (All India 2012)
Answer:
(i) The bond energy of F – F bond is lower than that of N-F bond so NF3 is an exothermic . compound. On the other hand bond energy
of Cl – Cl bond is higher than that of N – Cl bond so NCl3 is an endothermic as well as unstable compound.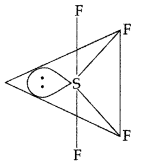
(ii) SF4 has trigonal bipyramidal structure in which one position of equitorial is occupied by a lone pair of electrons. Sulphur undergoes sp3d hybridisation and has sea-saw geometry.
Question 61.
Explain the following :
(a) Xenon does not form such fluorides as XeF3and XeF5.
(b) Out of noble gases, only Xenon is known to form real chemical compounds. (Comptt. Delhi 2012)
Answer:
(a) By impairing of one paired orbital, two singly occupied orbitals come into existence.Thus, either two or four or six singly occupied orbitals can be formed instead of one, three or five singly occupied orbitals. Hence XeF, XeF3 or XeF5 are not formed.
(b) Xe atom has a large size and lower ionisation potential and hence the force of nucleus over the electrons is weak and hence very small energy can excite the electrons and hence it is easier for Xenon to form compounds than other noble gases.
Question 62.
(a) Which form of sulphur shows paramagnetic behaviour and why ?
(b) Fluorine exhibits only -1 oxidation state whereas other halogens exhibit +1, +3, +5 or +7 oxidation states also. Explain as to why. (Comptt All India 2012)
Answer:
(a) In vapour state sulphur partly exists as S2molecule which has two unpaired electrons in the antibonding π* orbitals like O2 and hence exhibits paramagnetism.
(b) It is because fluorine is the most electronegative element and it does not have d-orbitals.
Question 63.
How is XeO3 obtained? Write the related chemical equations. Draw the structure of XeO3 (Comptt. All India 2012)
Answer:
Hydrolysis of XeF4 and XeF6 with water gives XeO3
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2
XeF6 + 3H2O → XeO3 + 6HF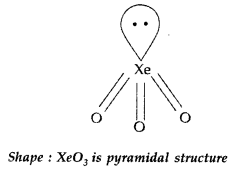
Question 64.
What happens when
(i) PCl5 is heated? (ii) H3PO3 is heated? Write the reactions involved. (Delhi 2013)
Answer:
(i) On heating PCl5 decomposes into PCl3 + Cl2![]()
(ii) Orthophosphorous acid on heating disproportionates to give orthophosphoric acid and phosphine.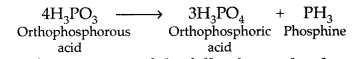
Question 65.
Draw the structures of the following molecules: (All India 2013)
(i) XeOF4 (ii) H3PO3
Answer:
(i) XeOF4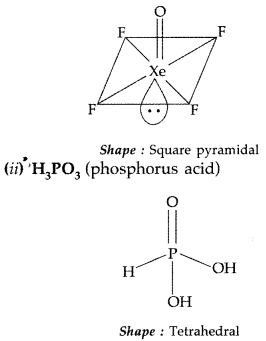
Question 66.
How are interhalogen compounds formed? What general compositions can be assigned to them? (All India 2013)
Answer:
Interhalogen compounds: Halogens react with each other to form a number of compounds called interhalogen compounds, whose general formula is XX’n.
Where X = less electronegative atom (have larger size)
X’ = more electronegative atom (have smaller size)
n = no. of more electronegative atoms/high
They are of four types :
XX’ = ClF, BrF, IF, BrCl, ICl, IBr
XX’3 = ClF3 BrF3, IF3, ICl3
XX’5 = ClF5, BrF5, IF5
XX’7 = IF7
Naming: The halogen with positive oxidation state named first and with negative oxidation state named after first with suffix ‘ide’.
Example : BrCl3 → Bromine trichloride
IF7 → Iodine heptafluoride
Preparation of Interhalogen Compounds :
By direct combination ;
Example: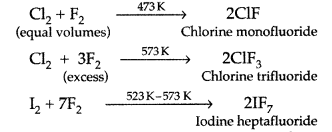
Question 67.
Draw the structures of the following molecules :
(i) XeF6 (ii) H2S2O7 (All India 2013)
Answer:
(i) XeF6 :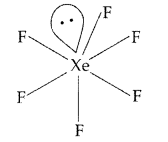
(ii) H2S2O7 :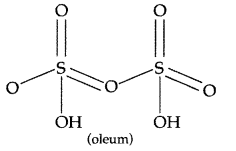
Question 68.
Draw the structures of the following molecules :
(i) N2O5 (ii) XeF2 (All India 2013)
Answer:
(i) N2O5: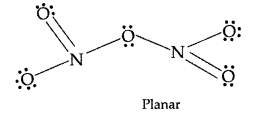
(ii) XeF2 :
Shape : Linear
Angle : F-Xe – F > 90°
Question 69.
Explain the following :
(a) NO2 readily forms a dimer.
(b) BiClj is more stable than BiCl5. (Comptt. Delhi 2013)
Answer:
(a) NO2 contains 7 + 2 × 8 i.e. 23 odd electrons. In the valence shell N has seven electrons and hence less stable. To acquire stability it dimerizes to form N2O4
(b) BiCl3 is more stable than BiCl5 due to inert pair effect because as we move down the group, the stability of +3 oxidation state increases and of +5 decreases.
Question 70.
Complete the following chemical equations :
(i) Ca3P2 + H2O →
(ii) Cu + H2SO4 (cone.) → (Delhi 2014)
Answer:
(i) Ca3P2 + 6H2O → 2PH3 + 3Ca(OH)2
(ii) Cu + 2H2SO4 (cone.) → CuS04 + 2H2O + SO2
Question 71.
Arrange the following in the order of property indicated against each set :
(i) HF, HCl, HBr, HI – increasing bond dissociation enthalpy.
(ii) H2O, H2S, H2Se, H2Te – increasing acidic character. (Delhi 2014)
Answer:
(i) HI < HBr < HCl < HF
(ii) H2O < H2S < H2Se < H2Te
Question 72.
Complete the following equations :
(i) P4 + H2O →
(ii) XeF4 + O2F2 → (All India 2014)
Answer:
(i) P4 + 6H2O → 2PH3+ 2H3PO3
(ii) XeF4 + O2F2
Question 73.
Draw the structures of the following :
(i) XeF2 (ii) BrF3 (All India 2014)
Answer:
(i) XeF2 : Refer to Q. 84 (ii), Page 125
(ii) BrF3 (SP3d hybridization)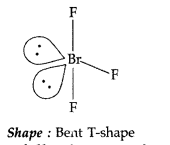
Question 74.
Complete the following equations :
(i) Ag + PCl5 →
(ii) CaF2 + H2SO4 → (All India 2014)
Answer:
(i) Ag + PCl5 → 2AgCl + PCl3
(ii) CaF2 + H2SO4 → CaSO4+ 2HF
Question 75.
Draw the structures of the following :
(i) XeF4
(ii) HClO4 (All India 2014)
Answer:
(i) XeF4: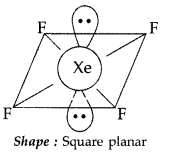
(ii) HClO4: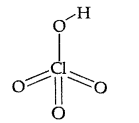
Question 76.
Complete the following equations :
(i) C + conc. H2SO4 →
(ii) XeF2 + H2O → (All India 2014)
Answer: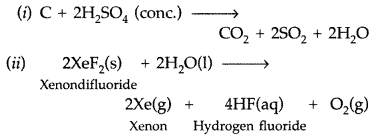
Question 77.
Draw the structures of the following :
(i) XeO3 (ii) H2SO4 (All India 2014)
Answer:
(i) XeO3 :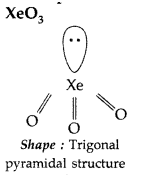
(ii) H2SO4: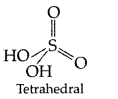
Question 78.
Draw the structure of each of the following:
(i) H2SO4
(ii) Solid PCl5 (Comptt. Delhi 2014)
Answer:
(i) H2SO4:
(ii) PCl5 (s):
Question 79.
Draw the structures of the following compounds :
(i) H2SO3
(ii) N2O5 (Comptt. Delhi 2014)
Answer: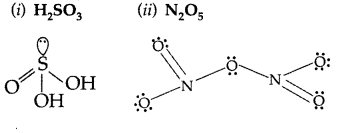
Question 80.
Complete the following chemical equations :
(i) PCl5
(ii) NaHCO3 + HCl → (Comptt. Delhi 2014)
Answer: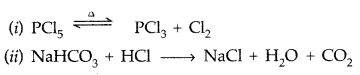
Question 81.
Complete the following chemical equations : (Comptt. All India 2014)
(i) SO2 + MnO4– + H2O →
(ii) F2 (g) + H2O (l) →
Answer: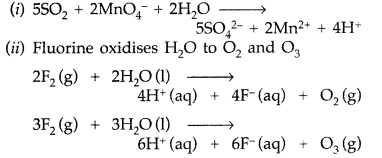
Question 82.
Complete the following chemical reaction equations : (Comptt. All India 2014)![]()
Answer: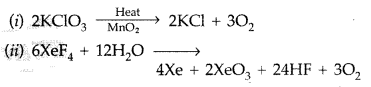
Question 83.
Complete the following chemical equations : (Comptt. All India 2014)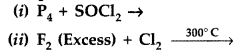
Answer: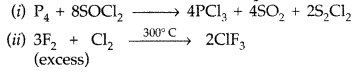
Question 84.
Draw the structures of the following :
(i) H2SO4
(ii) XeF2 (Comptt. Delhi 2015)
Answer: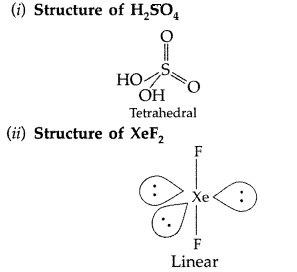
Question 85.
Explain the following :
(i) Nitrogen is much less reactive than phosphorus.
(ii) NF3 is an exothermic compound but NCl3 is an endothermic compound. (Comptt All India 2015)
Answer:
(i) Due to presence of weak single bond in P – P than N = N, phosphorous is more reactive than nitrogen and also because of high bond dissociation enthalpy of N = N.
(ii) Due to smaller size of F as compared to Cl, the N – F bond is much stronger than N – Cl bond while bond dissociation energy of F2 is much lower than that of Cl2. Therefore, energy released during the formation of NF3 molecule is more than the energy needed to break N2 and F2 molecules into individual atoms. In other words, formation of NF3 is an exothermic reaction.
The energy released during the formation of NCl3molecule is less than the energy needed to break N2 and Cl2 molecules into individual atoms. Thus formation of NCl3 is an endothermic reaction.
Question 86.
What happens when:
(i) SO2 gas is passed through an aqueous solution of Fe3+ salt?
(ii) XeF4 reacts with SbF5? (All India 2016)
Answer:
(i) In this sulphur dioxide acts as a reducing agent and reduces Fe3+ to Fe2+.
2Fe3+ + SO2 + 2H2O → 2Fe2+ + SO42- + 4H+
(ii) XeF4 + SbF5 → [XeF3]+ [SbF6]–
Question 87.
Draw the structures of the following compounds
(i) BrF3 (ii) XeF4 (All India, Comptt. Delhi 2016)
Answer: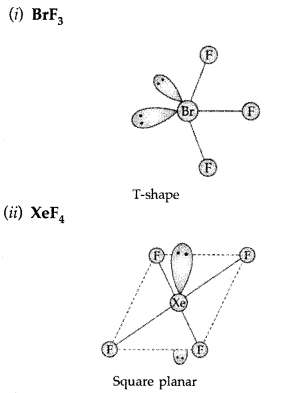
Question 88.
What happens when :
(i) Concentrated H2SO4 is added to calcium fluoride?
(ii) SO3 is passed through water? (Comptt. All India 2016)
Answer:
(i) Cone. H2SO4 reacts with CaF2 giving Hydrogen Fluoride
CaF2 + H2SO4 → CaSO4 + 2HF
(ii) SO3 passed in water giving Sulphuric Acid
SO3 + H2O → H2SO4
Question 89.
Complete the following reactions:
(i) NH3 + 3CI3 (excess) →
(ii) XeF6 + 2H2O → (Delhi 2017)
Answer: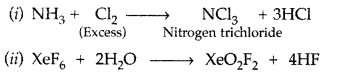
Question 90.
What happens when H3PO3 is heated? (Delhi 2017)
Answer:![]()
Question 91.
Draw the structures of the following:
(i) H2S2O7 (ii) XeF6
Answer:
(i) H2S2O7 (Pyrosulphuric acid or Oleum)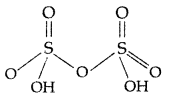
(ii)
Question 92.
Draw the structures of the following:
(i) H3PO2 (ii) XeF4 (Delhi 2017)
Answer: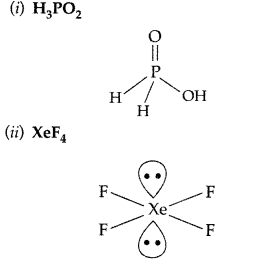
Question 93.
Complete the following reactions: (Delhi 2017)
(i) Cl2 + H2O →
(ii) XeF6 + 3H2O →
Answer:
(i) Cl2 + H2O → [HCl + HOCl] → 2HCl + [O]
(ii) XeF6 + 3H2O → XeO3 + 6HF
Question 94.
What happens when
(i) cone. H2SO4 is added to Cu?
(ii) SO3 is passed through water? Write the equations. (Delhi 2017)
Answer:
(i) Cu + 2H2SO4 (cone) → CuSO4 + 2H2O+SO2
(ii) SO3 + H2O → H2SO4
Question 95.
Draw the structures of the following:
(i) H4P2O7
(ii) XeOF4
Answer:
(i) H4P2O7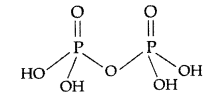
(ii)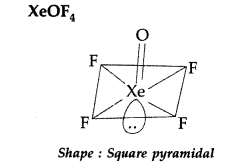
Question 96.
Complete the following chemical equations:
(i) F2 + 2Cl– →
(ii) 2XeF2 + 2H4O → (Delhi 2017)
Answer:
(i) F2+ 2Cl– → Cl2 + 2F–
(ii) 2XeF2 + 2H2O → 2Xe + 4HF + O2
Question 97.
What happens when
(i) HCl is added to MnO2?
(it) PCl5is heated? Write the equations involved. (Delhi 2017)
Answer:
(i) When HCl is added to MnO2, chlorine gas is formed, along with other products.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
(ii) PCl5
Question 98.
Draw the structures of the following:
(i) H2SO3 (ii) HClO3 (All India 2017)
Answer:
(i) Structure of H2SO3 (Sulphurous acid):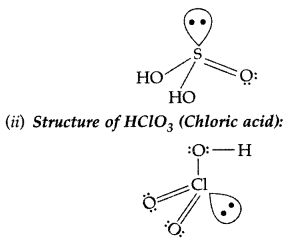
Question 99.
Draw the structures of the following:
(a) H2S2O8 (b) ClF3 (All India 2017)
Answer:
(a) Structure of H2S2O8: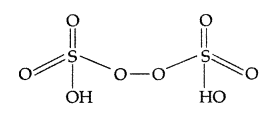
Question 100.
Draw the structures of the following:
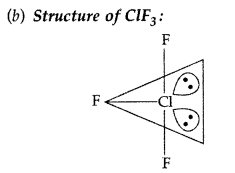
(a) XeF4 (b) BrF5 (All India 2017)
Answer:
(i) Structure of BrF5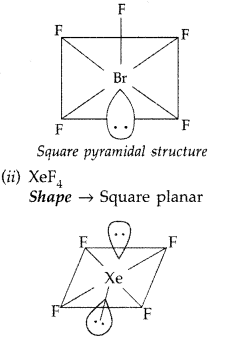
Question 101.
“Orthophosphoric acid (H3PO4) is non-reducing whereas hypophosphorus acid (H3PO2) is a strong reducing agent.” Explain and justify the above statement with suitable example. (Comptt. Delhi 2017)
Answer:
Orthophosphoric add (H3PO4) is not a reducing agent because it doesn’t contain any P-H bond whereas hypophosphorus acid (H3PO2) is a strong reducing agent as it contains two P-H bonds. H3PO2 can reduce silver nitrate (AgNO3) into metallic silver which H3PO4 can not.
4AgNO3 + H3PO2 + 2H2O → 4Ag ↓ + H3PO4 + 4HNO3
Question 102.
(a) What is the covalence of nitrogen in N2O5?
(b) BiH3 is a stronger reducing agent than SbH3, why? (Comptt. Delhi 2017)
Answer:
(a) The covalency of nitrogen in N2O5 is 4 because each nitrogen atom has four shared pairs of electrons.
(b) BiH3: Because it is a stronger reducing agent as its tendency to liberate H is maximum.
Question 103.
Account for the following:
(i) The two oxygen-oxygen bond lengths in ozone molecule are identical.
(ii) Most of the reactions of fluorine are exothermic. (Comptt. Delhi 2017)
Answer:
(i) Due to resonance the two oxygen atoms have partial double bond character and thus have same bond length i.e. 128 pm
(ii) Due to much higher electrode potential, high electro-negativity and low bond dissociation enthalpy of F2.
Question 104.
Account for the following :
(i) Two S-O bond lengths in SO2 are equal.
(ii) Fluorine shows only -1 oxidation state in its compounds. (Comptt. Delhi 2017)
Answer:
(i) Due to resonance in SO2 the double bond (π) electrons are distributed equally in both resonating structures as a result of which the bond length of two S-O becomes equal.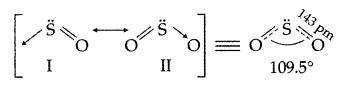
Because it is most electronegative element and does not have d-orbitals for octet expansion, therefore it shows only a negative oxidation state of -1.
Question 105.
Account for the following:
(i) Bond angle is
(ii) ICl is more reactive than I2. (Comptt. Delhi 2017)
Answer:
(i) Because in
(ii) Because I-Cl bond is weaker than I-1 bond as a result of which ICl breaks easily to form halogen atoms which readily bring about the reaction, hence more reactive.
Question 106.
“Orthophosphoric acid (H3PO4) is not a reducing agent whereas hypophosphorus acid (H3PO2) is a strong reducing agent.” Explain and justify the above statement with the help of a suitable example. (Comptt. All India 2017)
Answer:
Orthophosphoric add (H3PO4) is not a reducing agent because it doesn’t contain any P-H bond whereas hypophosphorus acid (H3PO2) is a strong reducing agent as it contains two P-H bonds. H3PO2 can reduce silver nitrate (AgNO3) into metallic silver which H3PO4 can not.
4AgNO3 + H3PO2 + 2H2O → 4Ag ↓ + H3PO4 + 4HNO3
Question 107.
Account for the following :
(i) NH3 is a stronger base than PH3.
(ii) Sulphur has a greater tendency for catenation than oxygen.
(iii) Bond dissociation energy of F2 is less than that of Cl3. (Delhi 2009)
Answer:
(i) Since both P and N contain lone pairs of electrons but due to small size and high electronegativity of Nitrogen in NH3, the electron density is much higher than PH3, therefore it can easily donate electrons and acts as strong Lewis base than PH3.
(ii) The greater catenation tendency of sulphur is due to two reasons :
(a) The lone pair of electrons feels more repulsion in 0-0 bond than S-S bond due to its small size and thus S-S forms strong bond.
(b) As the size of atom increases down the group from O – PO, the strength of bond increases and therefore catenation tendency also increases.
(iii) Due to smaller size of F than Cl as a result of which electron-electron repulsions between the lone pairs of electrons are very large than that of Cl, hence bond dissociation energy of F2 is less than that of Cl2.
Question 108.
Explain the following situations :
(i) In the structure of HNO3 molecule, the N-O bond (121 pm) is shorter than N – OH bond (140 pm).
(ii) SF4 is easily hydrolysed whereas SF6 is not easily hydrolysed.
(iii) XeF2 has a straight linear structure and not a bent angular structure. (Delhi 2009)
Answer:
(i)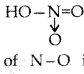
In the structure the bond length of N-O is shorter due to formation of coordinate bond and double bond while in N-OH the bond is single covalent due to which its bond length is greater than other N-O bond.
(ii) In SF4, due to less steric hindrance by four F atoms, H2O molecules can attack easily while in SF6 tire S atom is completely protected by six F atoms and does not allow H2O molecules to attack the S atom.
(iii) In XeF2 there are 2 bond pairs and 3 lone pairs and thus show sp3 d hybridization. It has linear geometry.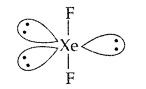
Question 109.
Explain the following observations :
(i) Fluorine does not exhibit any positive oxidation state.
(ii) The majority of known noble gas compounds are those of Xenon.
(iii) Phosphorus is much more reactive than nitrogen. (Delhi 2009)
Answer:
(i) Because it is most electronegative element and does not have d-orbitals for octet expansion, therefore it shows only a negative oxidation state of -1.
(ii) Because xenon has least ionization energy among noble gases and hence it readily forms chemical compounds particularly with oxygen and fluorine.
(iii) Because P-P single bond is much weaker than N = N triple bond and the bond length of nitrogen is small and bond dissociation energy is very large which makes it inert and unreactive and thus phosphorus becomes more reactive.
Question 110.
How would you account for the following :
(i) NCl3 is an endothermic compound while NF3 is an exothermic one.
(ii) XeF2 is a linear molecule without a bend.
(iii) The electron gain enthalpy with negative sign for fluorine is less than that for chlorine, still fluorine is a stronger oxidising agent than chlorine. (All India 2010)
Answer:
(i) F is more electronegative than Cl. The difference in the electronegativity between N and F is much more than the difference between electronegativity of N and Cl. So there is need of much more energy to break the N-F bond.
(ii) In XeF2 there are 2 bond pairs and 3 lone pairs and thus show sp3 d hybridization. It has linear geometry.
(iii) Because of small size of flourine atom and strong electron-electron repulsions in its compact 2p orbitals.
Question 111.
How would you account for the following :
(i) The electron gain enthalpy with negative sign is less for oxygen than that for sulphur.
(ii) Phosphorus shows greater tendency for catenation than nitrogen.
(iii) Fluorine never acts as the central atom in polyatomic interhalogen compounds. (All India 2010)
Answer:
(i) The least negative electron gain enthalpy of oxygen is due to small size and more interelectronic repulsion with coming electron.
(ii) The bond strength of P-P is more than N-N, therefore phosphorus shows more tendency for catenation than nitrogen.
(iii) Because F being smaller, it cannot accomodate larger sized other halogen atoms around it. Due to the absence of d-orbitals, F does not show positive oxidation state of +3, +5, +7 needed for the formation of polyatomic interhalogen compounds.
Question 112.
How would you account for the following :
(i) H2S is more acidic than H2O.
(ii) The N-O bond in
(iii) Both O2 and F2 stabilize high oxidation states but the ability of oxygen to stabilize the higher oxidation state exceeds that of fluorine. (All India 2011)
Answer:
(i) Since the size of sulphur is more than oxygen, S-H bond length increases and hence bond dissociation energy of S-H is less than O-H. Therefore S-FI easily loses H+ and thus is more acidic than H2O.
(ii) The resonating structure of
Answer:
(iii) Oxygen stabilizes the highest oxidation state even more than fluorine.
Example : Highest fluoride of Mn is MnF4 whereas highest oxide is Mn2O7. It is due to ability of oxygen to form multiple bonds with the metal atoms.
Question 113.
How would you account for the following :
(i) NF3 is an exothermic compound but NCl3 is not.
(ii) The acidic strength of compounds increases in the order :
PH3 < H2S < HCl.
(iii) SF6 is kinetically inert. (All India 2011)
Answer:
(i) F is more electronegative than Cl. The difference in the electronegativity between N and F is much more than the difference between electronegativity of N and Cl. So there is need of much more energy to break the N-F bond.
(ii) As the electronegativity increases in the same period from left to right so their electronegativity are in the increasing order, P < S < Cl.
In the same way the acid strength is also in the increasing order i.e. PH3 < H2S < HCl.
(iii) Because SF6 is showing steric hindrance due to 6 (six) fluorine atoms which make it unable to react further with any other atom.
Question 114.
Give reasons for the following:
(i) Where R is an alkyl group, R3P = O exists but R3N = O does not.
(ii) PbCl4 is more covalent than PbCl2.
(iii) At room temperature, N2 is much less reactive. (All India 2013)
Answer:
(i) Due to presence of d-orbitals in P, it can form pπ -dπ bonds and can extend its covalency beyond 4 while N cannot do so due to absence of d-orbitals.
(ii) According to Fajan’s rule, highly charged Pb4+can polarise the anion i.e., Cl – more effectively than Pb2+ and hence PbCl4 becomes more covalent than PbCl2.
(iii) Due to presence of triple bonds between 2 N atoms, their bond length decreases and hence bond dissociation energy increases which makes N2 lesser reactive. While in phosphorus due to presence of single bond, more bond length, bond dissociation energy is low, hence very reactive.
Question 115.
Give reasons for the following :
(i) Though nitrogen exhibits +5 oxidation state, it does not form pentahalide.
(ii) Electron gain enthalpy with negative sign of fluorine is less than that of chlorine.
(iii) The two oxygen-oxygen bond lengths in ozone molecule are identical. (All India 2013)
Answer:
(i) Due to absence of empty d-orbitals, N2 does not form pentahalide.
(ii) Because of small size of flourine atom and strong electron-electron repulsions in its compact 2p orbitals.
(iii) Due to resonance the two oxygen atoms have partial double bond character and thus have same bond length i.e. 128 pm
Question 116.
(a) Name the gas evolved on heating ammonium nitrate. Write the chemical reaction.
(b) Write two uses of ammonium nitrate. (Comptt. All India 2013)
Answer:
The gas evolved on heating is Nitrous oxide![]()
(b) Uses of NH4NO3
- It is used in fertilizers.
- It is used in explosives.
Question 117.
Account for the following :
(i) NF3 is an exothermic compound but NCl3 is an endothermic compound.
(ii) HF is not stored in glass bottles but is kept in wax-coated bottles.
(iii) Bleaching of flowers by Cl2 is permanent while that of SO2 is temporary. (Comptt. All India 2013)
Answer:
(i) F is more electronegative than Cl. The difference in the electronegativity between N and F is much more than the difference between electronegativity of N and Cl. So there is need of much more energy to break the N-F bond.
(ii) HF is highly corrosive and etches glass hence it is kept in wax-coated bottles.
(iii) Chlorine bleaches the material by oxidation hence it is permanent while SO2 bleaches the material by reduction and as the material is exposed to air, it gets oxidised and the colour is restored, hence it is temporary.
Question 118.
(a). With the help of chemical equations explain the principle of contact process in brief for the manufacture of sulphuric acid by contact process.
(b) Bismuth is a strong oxidizing agent in the pentavalent state. Explain. (Comptt. All India 2013)
Answer:
(a) Contact Process : Burning sulphur in an excess of air
S + O2 → SO2 (g)
or, By heating sulphide ores like pyrites in an excess of air :
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
In either case, an excess of air is used so that the SO2 produced is already mixed with oxygen for the next stage. This is reversible reaction and the formation of SO3 is exothermic in the presence of catalyst V2O5 at 720 K
2SO2 + O2 ⇌ 2SO3 ΔH = -196 KJ/mol
This cannot be done by simply adding water to the S03. The reaction is so uncontrollable that it creates a fog of H2S04. Instead, the S03 is first dissolved in cone. H2S04.
H2SO4 + SO3 → H2S2O7
The product is known as fuming sulphuric acid or oleum to which water is added to get H2SO4
H2S2O7 + H2O → 2H2SO4
(b) The stability of +5 oxidation state decreases and that of +3 state increases due to inert pair effect down the group therefore Bi (V) accepts two electrons and gets reduced to Bi (III).
Bi5+ + 2e– → Bi3+ :
So, Bi(V) is more stronger oxidising agent.
Question 119.
(a) Draw the structures of the following molecules :
(i) XeOF4 (ii) H2SO4
(b) Write the structural difference between white phosphorus and red phosphorus. (Delhi 2014)
Answer:
(a)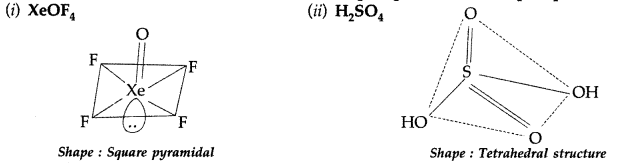
(b) White phosphorus exists as discrete P4 units with SP3 hybridized phosphorus atom, arranged tetrahedrally but in red phosphorus all P4tetrahedral units are linked with each other to form polymeric structure.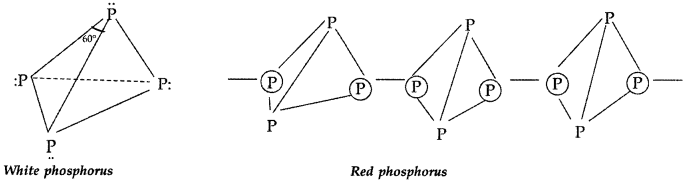
Question 120.
Account for the following :
(i) PCl5 is more covalent than PCl3.
(ii) Iron on reaction with HCl forms FeCl2 and not FeCl3.
(iii) The two 0-0 bond lengths in the ozone molecule are equal. (Delhi 2014)
Answer:
(i) In PCl5, phosphorus has +5 oxidation state and has less tendency to loose electrons than in +3 of PCl3. Therefore, PCl5 has more tendency to share e-1s than PCl3.
(ii) Because HCl on reaction with iron liberates H2gas which prevents the formation of ferric chloride.
(iii) Due to resonance the two oxygen atoms have partial double bond character and thus have same bond length i.e. 128 pm
Question 121.
Account for the following :
(i) Bi(V) is a stronger oxidizing agent than Sb(V).
(ii) N – N single bond is weaker than P – P single bond.
(iii) Noble gases have very low boiling points. (Delhi 2014)
Answer:
(i) Bi(V) is a stronger oxidizing agent than Sb(V) due to inert pair effect as the stability of lower oxidation state (+3) increases down the group.
(ii) Due to smaller size of Nitrogen, their lone pairs repel the bond pair of N – N bond while P – P due to bigger size does not show more repulsion.
(iii) Due to presence of weak Van der waal forces of attraction, noble gases have very low boiling point.
Question 122.
Account for the following :
(i) Sulphur in vapour form exhibits paramagnetic behaviour.
(ii) SnCl4 is more covalent than SnCl2.
(iii) H3PO2 is a stronger reducing agent than H3PO<sub3. (Delhi 2014)
Answer:
(i) In vapour state sulphur partly exists as S2 molecule which has two unpaired electrons in the antibonding II orbitals and hence exhibits paramagnetism.
(ii) Sn+4 in SnCl4 has more polarising power than SnCl2
(iii) H3PO2 contains two P-H bonds while H3PO3contains only one P-H bond therefore H3PO2 is stronger reducing agent.
Question 123.
Give reasons for the following :
(i) (CH3)3 P = O exists but (CH3)3 N = O does not.
(ii) Oxygen has less electron gain enthalpy with negative sign than sulphur.
(iii) H3PO2 is a stronger reducing agent than H3PO3. (All India 2014)
Answer:
(i) (CH3)3P = 0 exists due to presence of empty d-orbitals and thus can expand its covalency upto 6 but (CH3)3 N = O cannot expand its covalency due to absence of d-orbitals.
(ii) The least negative electron gains enthalpy of oxygen is due to small size and more interelectronic repulsion with coming electron.
(iii) H3PO2 contains two P-H bonds while H3PO3contains only one P-H bond therefore H3PO2 is a stronger reducing agent.
Question 124.
Give reasons:
(i) SO2 is reducing while TeO2 is an oxidizing agent.
(ii) Nitrogen does not form pentahalide.
(iii) ICl is more reactive than I2. (All India 2016)
Answer:
(i) SO2 is reducing while TeO2 is an oxidising agent because sulphur can expand its covalency upto +6 from +4 due to presence of empty d-orbital but as we move down the group the stability of +6 oxidation state decreases and of +4 oxidation state increases due to inert pair effect. Hence SO2acts as reducing agent while TeO2 acts as an oxidising agent.
(ii) Due to absence of empty d-orbitals, N2 does not form pentahalides.
(iii) Because ICl bond is weaker than I -I bond as a result of which ICl breaks easily to form halogen atoms which readily bring about the reaction, hence more reactive.
Question 125.
Give reasons:
(i) Thermal stability decreases from H2O to H2Te.
(ii) Fluoride ion has higher hydration enthalpy than chloride ion.
(iii) Nitrogen does not form pentahalide. (Delhi 2017)
Answer:
(i) Thermal stability decreases from H2O to H2Te due to weakening of bond between hydrogen and the atom from O to Te as size is increasing down the group.
(ii) Fluoride ion has higher hydration enthalpy than chloride ion due to stronger attractions of smaller in size fluoride ion.
(iii) Nitrogen does not contain’d’ orbitals.
Question 126.
Give reasons for the following:
(a) Red phosphorus is less reactive than white phosphorus.
(b) Electron gain enthalpies of halogens are largely negative.
(c) N2O5 is more acidic than N2O3.(All India 2017)
Answer:
(a) Red phosphorus is less reactive than white phosphorus because white phosphorus possess angle strain where long angles are only 60° making it more reactive. Also, red phosphorus being polymeric is less reactive than white phosphorus which has discrete tetrahedral structure.
(b) Electron gain enthalpies of halogens are largely negative due to high effective nuclear charge and smaller size among period. They readily accept an electron to attain noble gas configuration.
(c) N2O5 is more acidic than N2O3 because higher the oxidation state, higher will be acidic character. N2O5 has +5 oxidation state and N2O3 has +3 oxidation state.
Question 127.
(a) Arrange the hydrides of group 16 in increasing order of their acidic character. Justify your answer.
(b) Draw structure of XeOF4. (Comptt. Delhi 2017)
Answer:
(a) H2O < H2S < H2Se < H2Te
As we move down the group the bond dissociation enthalpy decreases due to increase in bond length and size of the central atom.
(b)
Question 128.
(a) Account for the the following :
(i) PCl5 is more covalent than PCl3
(ii) Iron on reaction with HC1 forms FeCl2 and not FeCl3
(b) Draw structure of XeO3 (Comptt. Delhi 2017)
Answer:
(a) (i) In PCl5, phosphorus has +5 oxidation state and has less tendency to loose electrons than in +3 of PCl3. Therefore, PCl5 has more tendency to share e-1s than PCl3.
(ii) Because HCl on reaction with iron liberates H2gas which prevents the formation of ferric chloride.
(iii) Due to resonance the two oxygen atoms have partial double bond character and thus have same bond length i.e. 128 pm
(b)
Question 129.
(a) Draw the structures of the following :
(i) H2S2O8 (ii) HClO4
(b) How would you account for the following :
(i) NH3 is a stronger base than PH3
(ii) Sulphur has a greater tendency for catenation than oxygen.
(iii) F2 is a stronger oxidising agent than Cl2. (All India 2009)
Answer:
(a) (i) H2S2O8 (Peroxodisulphuric acid) or Marshall’s acid :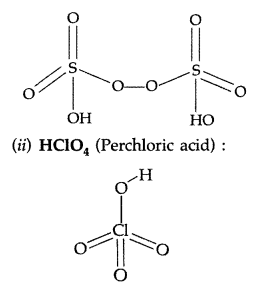
(b)
(i) Since both P and N contain lone pairs of electrons but due to small size and high electronegativity of Nitrogen in NH3, the electron density is much higher than PH3, therefore it can easily donate electrons and acts as strong Lewis base than PH3.
(ii) The greater catenation tendency of sulphur is due to two reasons :
(a) The lone pair of electrons feels more repulsion in 0-0 bond than S-S bond due to its small size and thus S-S forms strong bond.
(b) As the size of atom increases down the group from O – PO, the strength of bond increases and therefore catenation tendency also increases.
(iii) Due to low bond dissociation enthalpy and high electronegativity of Fluorine, it has strong tendency to accept electrons and thus get reduced.
F + e– → F–
Therefore F2 acts as strong oxidising agent, while Cl2 is weak oxidising agent due to low electronegativity.
Question 130.
(a) Draw the structures of the following :
(i) H2S2O7 (ii) HClO3
(b) Explain the following observations :
(i) In the structure of HNO3 the N-O bond (121 pm) is shorter than the N- OH bond (140 pm).
(ii) All the P-Cl bonds in PCl5 are not equivalent.
(iii) ICl is more reactive than I2. (All India 2009)
Answer:
(a) (i) H2S2O7 (Pyrosulphuric acid) or oleum :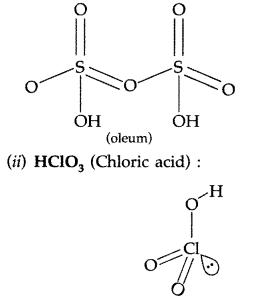
(b) (i) The N-O bond has partial double bond character while the N-OH bond is a single bond in both resonance of HNO3
(ii) All the P-Cl bonds in PCl5 are not equivalent due to the fact that the axial bond pairs suffer more repulsion as compared to equatorial bond pairs.
(iii) Because ICl bond is weaker than I-I bond as a result of which ICl breaks easily to form halogen atoms which readily bring about the reaction, hence more reactive.
Question 131.
(a) Draw the structures of the following :
(i) H3PO2 (ii) BrF3
(b) How would you account for the following observations :
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) Bond dissociation energy of fluorine is less than that of chlorine.
(iii) No chemical compound of helium is known. (All India 2009)
Answer:
(a) (i) H3PO2 (Hypophosphorous acid)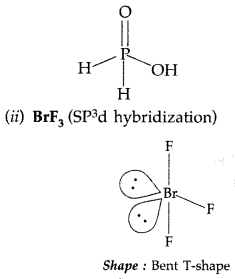
(b) (i) The bond strength of P-P is more than N-N, therefore phosphorous shows more tendency for catenation than nitrogen.
(ii) Due to smaller size of F than Cl as a result of which electron-electron repulsions between the lone pairs of electrons are very large than that of Cl, hence bond dissociation energy of F2 is less than that of Cl2.
(iii) Because the ionization energy of Helium is very high and the empty d-orbitals are also absent in it.
Question 132.
(a) Draw the structures of the following :
(i) N2O5 (ii) XeOF4
(b) Explain the following observations :
(i) The electron gain enthalpy of sulphur atom has a greater negative value than that of oxygen atom.
(ii) Nitrogen does not form pentahalides.
(iii) In aqueous solutions HI is a stronger acid than HCl. (All India 2009)
Answer:
(a) (i) N2O5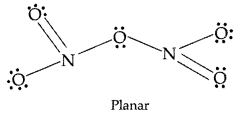
(ii) XeOF4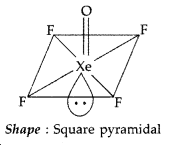
(b) (i) Because enthalpy of dissociation of S-S bond is higher than 0-0 bond and the hydration energy of S2- is less than that of O2- ion.
(ii) Due to absence of empty d-orbitals, N2 does not form pentahalides.
(iii) Due to lower bond dissociation energy and higher degree of ionization, HI acts as stronger acid than HCl in aqueous solution.
Question 133.
(a) Draw the structures of the following :
(i) XeF4 (ii) H2S2O7
(b) Explain the following observations :
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) The negative value of electron gain enthalpy is less for fluorine than that for chlorine.
(iii) Hydrogen fluoride has a much higher boiling point than hydrogen chloride. (All India 2009)
Answer:
(a) (i) XeF4 :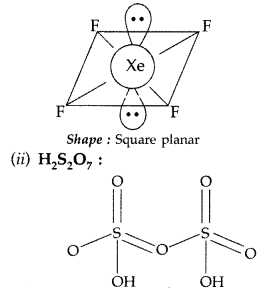
(b) (i) The bond strength of P-P is more than N-N, therefore phosphorous shows more tendency for catenation than nitrogen.
(ii) Because of small size of flourine atom and strong electron-electron repulsions in its compact 2p orbitals.
(iii) Hydrogen fluoride (HF) has higher boiling point than HC1 due to extensive intermolecular hydrogen bonding while HCl doesn’t show this H-bonding.
Question 134.
(a) Draw the structures of the following :
(i) PCl5(s)
(ii)
(b) Explain the following observations :
(i) Ammonia has a higher boiling point than phosphine.
(ii) Helium does not form any chemical compound.
(iii) Bi (V) is a stronger oxidising agent than Sb (V). (All India 2009)
Answer:
(a) (i) PCl5 (s)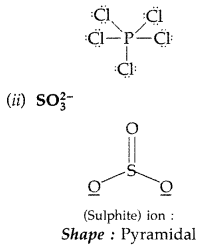
Angle: The angle O – S – O is greater than 90°
(b) (i) Due to intermolecular H-bonding in NH3 it has higher boiling point than PH3 which does not have any H-bonding.
(ii) Because the ionization energy of Helium is very high and very high positive electrons gain enthalpy.
(iii) The stability of +5 oxidation state decreases and that of +3 state increases due to inert pair effect down the group therefore Bi(v) accepts two electrons and gets reduced to Bi (v).
Bi5+ + 2e– → Bi3+
Question 135.
(a) Complete the following chemical equations :
(i) NaOH (aq) + Cl2 (g) →
(Hot and cone.)
(ii) XeF6 (s) + H2O(l) →
(b) How would you account for the following?
(i) The value of electron gain enthalpy with negative sign for sulphur is higher than that for oxygen.
(ii) NF3 is an exothermic compound but NClj is endothermic compound.
(iii) ClF3 molecule has a T-shaped structure and not a trigonal planar one. (Delhi 2010)
Answer:
(a) (i) 6NaOH + 3Cl2 → 5NaCl + 1NaClO3 + 3H2O
(ii) XeF6 (s) + 3H2O (1) → XeO3 (s) + 6HF (aq)
(b) (i) Because of enthalpy of dissociation of S- S bond is higher than O-O bond and the hydration energy of S2- is less than that of O2- ion.
(ii) Due to smaller size of F as compared to Cl, the N – F bond is much stronger than N-Cl bond while bond dissociation energy of F2 is much lower than that of Cl2. Therefore, energy released during the formation of NF3 molecule is more than the energy needed to break N2 and F2 molecules into individual atoms. In other words, formation of NF3is an exothermic reaction.
The energy released during the formation of NCl3molecule is less than the energy needed to break N2 and Cl2 molecule into individual atoms. Thus formation of NCl3 is an endothermic reaction.
(iii) The electronic configuration of Cl is 1s2 2s22p6 3s2 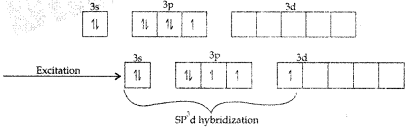
Question 136.
(a) Complete the following chemical reaction equations :
(i) P4 + SO2Cl2 →
(ii) XeF4 + H2O →
(b) Explain the following observations giving appropriate reasons :
(i) The stability of +5 oxidation state decreases down the group in group 15 of the periodic table.
(ii) Solid phosphorus pentachloride behaves as an ionic compound.
(iii) Halogens are strong oxidizing agents. (Delhi 2010)
Answer:
(a)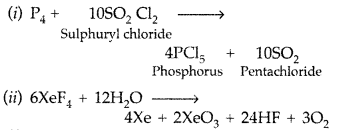
(b) (i) Group 15 first elements are pentavalent, therefore they can show positive oxidation state +3 (due to P-electron) and +5 (due to P and S electrons). In a group the +5 oxidation state stability decreases but +3 oxidation state increases due to inter pair effect which results the 5- orbitals electrons to participate in bonding hence shows +3 oxidation state as their stable oxidation state.
(ii) PCl5 conducts electricity in the molten state. This means that in solid state it exists as [PCl4]+[PCl6]– in which the cation is tetrahedral and the anion is octahedral.
2PCl5 → [PCl4]+ [PCl6]–
On melting, these ions become free to move and hence PCl5 conducts electricity in the molten state.
(iii) As halogens are strong elctron acceptors and change to negative ions and thus undergo reduction, so they are strong oxidising agent.
Question 137.
(a) Explain the following :
(i) NF3 is an exothermic compound ’ whereas NCl3is not.
(ii) F2 is most reactive of all the four common halogens.
(b) Complete the following chemical equations :
(i) C + H2SO4 (cone) →
(ii) P4 + NaOH + H2O →
(iii) Cl2 + F2 (excess) → (Delhi 2010)
Answer:
(a) (i) F is more electronegative than Cl. The difference in the electronegativity between N and F is much more than the difference between electronegativity of N and Cl. So there is need of much more energy to break the N-F bond.
(ii) Because of the low bond dissociation energy F2readily dissociates into atoms and reacts with other substances readily.
(b) (i) C + 2H2SO4 (cone.) → CO2 + 2SO2 + 2H2O
(ii) P4 + 3NaOH + 3H2O → PH2 + 3NaH2PO2
(iii) Cl2 + 3F2 (excess) → 2ClF3
Question 138.
(a) Account for the following :
(i) The acidic strength decreases in the order HCl > H2S > PH3
(ii) Tendency to form pentahalides
decreases down the group in group 15 of the
(b) Complete the following chemical equations :
(i) P4 + SO2Cl2 →
(ii) XeF2 + H2O →
(iii) I2 + HNO3 (conc) → (Delhi 2010)
Answer:
(a) (i) Because of decrease in electronegativity from chlorine to phosphorous, the bond dissociation enthalpy from HCl to H-P increases and their tendency to release H+ decreases and thus acidic strength decreases.
(ii) Down the group, the tendency of next ‘s’ orbital’s electron to jump to previous’d’ orbital decreases very much due to inert pair effect.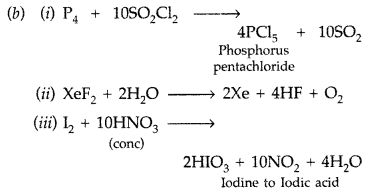
Question 139.
(a) Draw the structures of the following molecules :
(i) (HPO3)3
(ii) BrF3
(b) Complete the following chemical equations :
(i) HgCl2 + PH3 →
(ii) SO3 + H2SO4 →
(iii) XeF4 + H2O → (All India 2010)
Answer:
(a) (i) (HPO3)3 :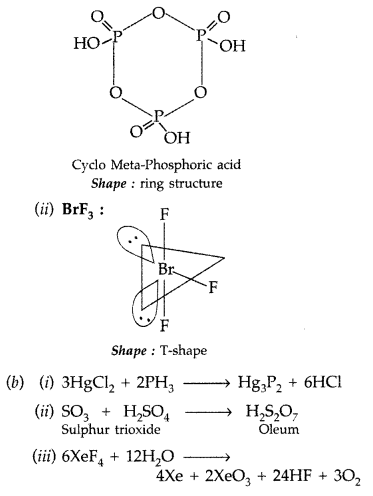
Question 140.
(a) What happens when
(i) chlorine gas is passed through a hot concentrated solution of NaOH?
(ii) sulphur dioxide gas is passed through an aqueous solution of a Fe (III) salt?
(b) Answer the following :
(i) What is the basicity of H3PO3 and why?
(ii) Why does fluorine not play the role of a central atom in interhalogen compounds?
(iii) Why do noble gases have very low boiling points? (All India 2010)
Answer: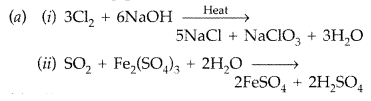
(b) (i) Basicity of H3PO3 = 2
Because basicity is the number of replaceable H+ions in an acid and H3PO3 is a Dibasic acid.
(ii) Because F being smaller, it cannot accomodate larger sized other halogen atoms around it. Due to the absence of d-orbitals, F does not show positive oxidation state of +3, +5, +7 needed for the formation of polyatomic interhalogen compounds.
(iii) Because the atoms of these elements are held together by weak van der Waal’s forces of attraction.
Question 141.
(a) Complete the following chemical reaction equations :
(i) P4 + SO2Cl2 →
(ii) XeF6 + H2O →
(b) Predict the shape and the asked angle (90° or more or less) in each of the following cases:
(i)
(ii) ClF3 and the angle F – Cl – F
(iii) XeF2 and the angle F – Xe – F (Delhi 2012)
Answer:
(a) (i) P4 + 10SO2Cl2 → 4PCl5 + 10SO2
or P4 + 8SO2Cl2 → 4PCl3 + 4SO2 + 2S2Cl2
(ii) XeF6 + H2O → XeOF4 + 2HF
or XeF6 + 2H2O → XeOF4 + 4HF
or XeF6 + 3H2O → XeO3 + 6HF
(b) (i) 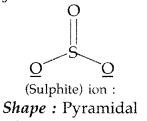
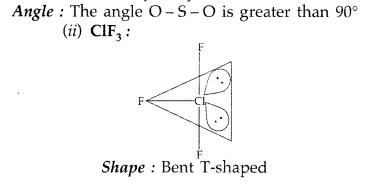
Angle : The angle F-Cl-F is lesser than 90°
(iii) XeF2; Structure :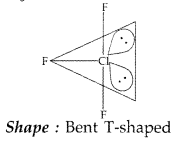
Shape : Linear
Angle ; F-Xe – F > 90°
Question 142.
(a) Complete the following chemical equations :
(i) NaOH (hot and cone.) + Cl2 →
(ii) XeF4 + O2F2 →
(b) Draw the structures of the following molecules :
(i) H3PO2 (ii) H2S2O7 (iii) XeOF4 (Delhi 2012)
Answer:
(a) (i) 3Cl2+ 6NaOH → 5 NaCl + NaClO3 + 3H2O
(ii) XeF4 + O2F2 → XeF6 + O2
(b) (i) H3PO2: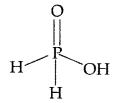
(ii) H2S2O7:
(iii) XeOF2:
Question 143.
(a) Draw the molecular structure of following compounds :
(i) XeF6 (ii) H2S2O8
(b) Explain the following observations :
(i) The molecules NH3 and NF3 have dipole moments which are of opposite direction.
(ii) All the bonds in PCl5 molecule are not equivalent.
(iii) Sulphur in vapour state exhibits paramagnetism. (Delhi 2012)
Answer:
(a) (i) XeF6: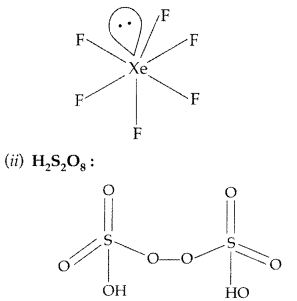
(b) (i) Because F is more electronegative than N in NF3 whereas N is more electronegative than H in NH3.
(ii) Because PCl5 has a trigonal bipyramidal structure in which the three equatorial P-Cl bonds are equivalent while the two axial bonds are longer than equatorial bonds.
(iii) Because sulphur in vapour state has two unpaired electrons in the antibonding π* orbitals like O2.
Question 144.
(a) Complete the following chemical equations
(i) XeF4 + SbF5 →
(ii) Cl2 + F2 (excess) →
(b) Explain each of the following :
(i) Nitrogen is much less reactive than phosphorus.
(ii) The stability of +5 oxidation state decreases down group 15.
(iii) The bond angles (O – N – O) are not of the same value in
Answer:
(a) (i) XeF4 + SbF5 → [XeF3]+ [SbF6]–
(ii) Cl2 + 3F2 (excess) → 2ClF3
(b) (i) Due to presence of triple bond between two nitrogen atoms/high bond dissociation enthalpy of nitrogen, whereas in phosphorus molecule there is single bond between four atoms of phosphorus so have low bond dissociation enthalpy.
(ii) Because the participation of outer s- electron pair goes on decreasing down the group due to inert pair effect.
(iii) Because
Question 145.
(a) Draw the molecular structures of the following compounds :
(i) N2O5 (ii) XeOF4
(b) Explain the following observations :
(i) Sulphur has a greater tendency for catenation than oxygen.
(ii) IC1 is more reactive than I2.
(iii) Despite lower value of its electron gain
enthalpy with negative sign, fluorine (F2) is a stronger oxidising agent than Cl2. (All India 2012)
Answer:
(a)
(i) N2O5
(ii) XeOF4
(b) (i) It is because S – S bond is stronger than 0-0 bonds as there is more interelectronic repulsion in O – O due to small size than in S – S.
(ii) ICl is more reactive than I2 because I-Cl bond is polar and weaker while I-I bond is stronger and non-polar.
(iii) It is due to (a) low enthalpy of dissociation of F – F bond, (b) high hydration enthalpy of F.
Question 146.
(a) Complete the following chemical equations :
(i) Cu + HNO3 (dilute) →
(ii) XeF4 + O2F2 →
(b) Explain the following observations :
(i) Phosphorus has greater tendency for catenation than nitrogen.
(ii) Oxygen is a gas but sulphur a solid.
(iii) The halogens are coloured. Why? (All India 2012)
Answer:
(a) (i) 3Cu + 8HNO3 (dil) → 3CU(NO3)2 + 2NO + 4H2O
(ii) XeF4 + O2F2 → XeF6 + O2
(b) (i) The self linking property or catenation property of nitrogen is less than that of phosphorus because N – N bond is weaker than P – P bond.
(ii) Oxygen forms a stable diatomic molecule. In 02 molecule two atoms of oxygen have joined together through double bond 0 = 0. The multiple bonding is possible due to small size of oxygen atoms. So oxygen is a gas. S has eight atoms arranged in the form of a puckered ring per molecule in which two sulphur atoms are joined by covalent bonds. So sulphur is a solid at room temperature.
(iii) All halogens are coloured. This is due to absorption of radiation in visible region which results in the excitation of outer electrons to higher energy level. So they display different colours.
Question 147.
(a) Draw the structures of the following molecules :
(i) H3PO2 (ii) ClF3
(b) Explain the following observations :
(i) Nitrogen is much less reactive than phosphorus.
(ii) Despite having greater polarity, hydrogen fluoride boils at a lower temperature than water.
(iii) Sulphur has greater tendency for catenation than oxygen in the same group. (All India 2012)
Answer:
(a) (i) H3PO2 :
(ii) ClF3 :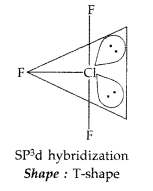
(b) (i) Because P-P bond is weaker than N ≡ N bond, N2 is inert due to the presence of triple bond.
(ii) In water O is larger than F therefore Van- der-Waals forces of attraction increase with increase in size and hence the boiling point increases.
(iii) It is because S – S bond is stronger than 0-0 bond as there is more interelectronic repulsion in O – O than in S-S.
Question 148.
(a) Draw the structures of the following molecules :
(i) N2O5 (ii) HClO4
(b) Explain the following observations :
(i) H2S is more acidic than H2O.
(ii) Fluorine does not exhibit any positive oxidation state.
(iii) Helium forms no real chemical compound. (All India 2012)
Answer:
(a) (i) N2O5 :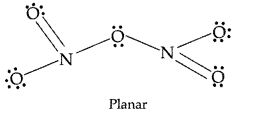
(ii) HClO4 :
(b) (i) H2S is more acidic than H2O because bond dissociation enthalpy of H – S bond in H2S is less than that of H – O bond in
H2O.
(ii) F is the most electronegative element. It has no d-orbitals and therefore, there is no scope for any electron promotion. So it can only show oxidation state of -1 in its compounds.
(iii) Helium has very high ionisation enthalpy and no vacant d-orbitals, therefore no chemical compound of helium is known.
Question 149.
(a) Describe the conditions and the steps involved in the manufacture of sulphuric acid by contact process. Write the necessary reactions. (No diagram is required)
(b) Give reasons :
ii) Bond dissociation energy of F2 is less than that of Cl2.
(ii) Nitric oxide becomes brown when released in air. (Comptt. Delhi 2012)
Answer:
(a) Contact Process : Burning sulphur in an excess of air
S + O2 → SO2 (g)
or, By heating sulphide ores like pyrites in an excess of air :
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
In either case, an excess of air is used so that the SO2 produced is already mixed with oxygen for the next stage. This is reversible reaction and the formation of SO3 is exothermic in the presence of catalyst V2O5 at 720 K
2SO2 + O2 ⇌ 2SO3 ΔH = -196 KJ/mol
This cannot be done by simply adding water to the S03. The reaction is so uncontrollable that it creates a fog of H2S04. Instead, the S03 is first dissolved in cone. H2S04.
H2SO4 + SO3 → H2S2O7
The product is known as fuming sulphuric acid or oleum to which water is added to get H2SO4
H2S2O7 + H2O → 2H2SO4
(b) (i) Bond dissociation energy of F2 is less than that of Cl2 because of large electron- electron repulsion among the lone pair in F2 molecule.
(ii) Nitric oxide becomes brown when released in air because of the formation of NO2 gas.
Question 150.
Account for the following :
(a) Thermal stability of water is much higher than that of H2S.
(b) White phosphorus is more reactive than red phosphorus.
(c) Ammonia acts as a ligand.
(d) Bismuth is a strong oxidizing agent in pentavalent state.
(e) Concentrated sulphuric acid is a strong dehydrating agent. (Comptt. Delhi 2012)
Answer:
(a) The thermal stablility of hydrides decrease from H2O to H2S because as the size of the atom increases, the bond becomes weaker and thus breaks on heating.
(b) Red phosphorus is regarded as a polymer consisting of chains of P4 tetrahedral linked together. This makes phosphorus denser and less reactive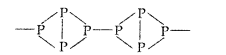
But in white phosphorus (P4), the four phosphorus atoms lie at the corners of a regular tetrahedron. Each phosphorus atom is linked to each of the other three atoms by covalent bond. The bond angle is equal to 60° which suggests that the molecule is under strain and hence active in nature.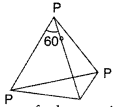
(c) Due to the presence of a lone pair of electrons on nitrogen atom, it has a tendency to donate an electron pair, hence acts as a ligand.
(d) In Bismuth, the inert pair effect is very prominent. Thus +5 oxidation state is less stable in comparison to +3 oxidation state i.e
it readily accepts two electrons in pentavalent state and gets reduced to trivalent state. Therefore, it acts as a strong oxidising agent.
(e) Cone. H2SO4 has great affinity for water molecule i.e it acts as a dehydrating agent.
Question 151.
Account for the following :
(i) Chlorine water loses its yellow colour on standing.
(ii) BrCl3 is more stable than BrCl5.
(iii) Fluorine does not form oxoacids.
(iv) PCl5 acts as an oxidising agent.
(v) SO2 is an air pollutant. (Comptt. All India 2012)
Answer:
(i) Cl2 water on standing loses its yellow colour due to the formation of HCl and HOCl. HOCl is unstable and decomposes to HCl. As a result, yellow colour disappears.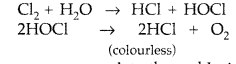
(ii) In BrCl3, Br can accommodate three chlorine atoms around it and hence it is stable but in BrCl5, five Cl atoms cannot be accommodated around Br and hence it is unstable.
(iii) Due to high electonegativity and small size F does not form oxoacids.
(iv) In PCl5, the oxidation state of phosphorus is +5. Thus, it cannot increase its oxidation state beyond +5. So PCl5 cannot act as a reducing agent. However, it can decrease its oxidation state from +5 to +3 or to some lower value. Thus, it acts as an oxidising agent.
(v) SO2 is a pungent and irritating gas. It acts as an air pollutant due to the following reasons:
- It causes throat and eye irritation as it is absorbed readily by respiratory’ tract.
- It combines with moisture forming sulphurous acid. It is then converted into H2SO4. Both these acids cause acid rain and destroy the marble, corrode metals, deteriorate fabrics, paper, leather etc.
Question 152.
(a) With the help of chemical equations explain the principle of contact process in brief for the manufacture of sulphuric acid.
(No diagram)
(b) Account for the following :
(i) Bond dissociation energy of F2 is less than that of Cl2
(ii) Nitric oxide (NO) becomes brown when released in air. (Comptt. All India 2012)
Answer:
(a) Principle of contact process : The process involves the oxidation of sulphur dioxide by air in the presence of a catalyst.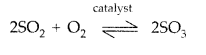
Sulphur trioxide is dissolved in 98% H2SO4 acid and oleum is formed
H2SO2 + SO3 → H2S2O7 (oleum)
Oleum is diluted with water to form sulphuric acid
H2S207 + H2O → 2H2S04
(b) (i) The low bond dissociation energy of F is due to high inter electronic repulsions between non-bonding electrons in 2p- orbitals as the size of F atoms is small. As a result, F-F bond is weaker than Cl-Cl bond.
(ii) It readily combines with 02 to form brown fumes of NO2.
2NO + O2→ 2NO2 (brown fumes)
Question 153.
(a) Give reasons for the following:
(i) Bond enthalpy of F2 is lower than that of Cl2.
(ii) PH3 has lower boiling point than NH3,
(b) Draw the structures of the following molecules:
(i) ClF3 (ii) (HPO3)3 (iii) XeF4 (Delhi 2013)
Answer:
(a) (i) Due to smaller size of F than Cl as a result
of which electron-electron repulsions between the lone pairs of electrons are very large than that of Cl, hence bond dissociation enthalpy of F2 is less than that of Cl2
(ii) PH3 has lower boiling point than NH3 because PH3 cannot form hydrogen bonds like NH3.
(b) (i) BrF3 :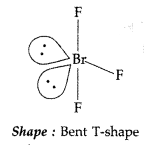
(ii) (HPO3)3: Cyclotrimetaphosphoric acid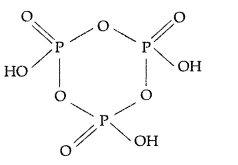
(iii) XeF4:
Question 154.
(a) Account for the following:
(i) Helium is used in diving apparatus.
(ii) Fluorine does not exhibit positive oxidation state.
(iii) Oxygen shows catenation behaviour less than sulphur.
(b) Draw the structures of the following molecules:
(i) XeF2 (ii) H2S2O8 (Delhi 2013)
Answer:
(i) Helium is used in diving apparatus because of its very low solubility in blood and therefore an oxygen-helium mixture is used for artificial respiration. (ii) Because it is most electronegative element and does not have d-orbitals for octet expansion, therefore it shows only a negative oxidation state of -1.
(ii) The greater catenation tendency of sulphur is due to two reasons :
(a) The lone pair of electrons feels more repulsion in 0-0 bond than S-S bond due to its small size and thus S-S forms strong bond.
(b) As the size of atom increases down the group from O – PO, the strength of bond increases and therefore catenation tendency also increases.
(b) (i) XeF2 :
(ii) H2S2O8 :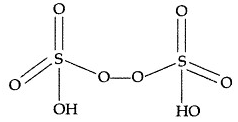
Question 155.
Give reasons for the following :
(i) Oxygen is a gas but sulphur is a solid.
(ii) O3 acts as a powerful oxidising agent.
(iii) BiH3 is the strongest reducing agent
amongst all the hydrides of Group 15 elements. (All India 2013)
Answer:
(i) Due to small size and high electronegativity, oxygen forms pπ – pπ multiple bonds and thus forms diatomic. O2 molecule are held together by weak van der Waals forces of attraction which can be easily overcome by collisions at room temperature. Therefore O2 is gas at room temperature. On the other hand due to higher tendency for catenation and lower tendency for pπ – pπ multiple bonds, intra-atomic S8 has strong forces of attraction which cannot be overcome by collisions. Therefore S is solid at room temperature.
(ii) O3 is a powerful oxidising agent due to its high energy content than oxygen and hence decomposes to give diatomic oxygen and atomic oxygen![]()
(iii) As we move down the group, the E—H bond length increases and their strength decreases. Bi—H bond is the weakest. It can break easily and evolves H2 gas which acts as the reducing agent.
Question 156.
(a) Write the balanced chemical equations for obtaining XeO3 and XeOF4 from XeF6.
(b) Account for the following :
(i) H2S is less acidic than H2Te.
(ii) H<sub3PO2 has reducing nature.
(iii) SO2 is an air pollutant. (Comptt. Delhi 2013)
Answer:
(a) XeF6 + 3H2O → XeO3 + 6HF
XeF6 + H2O → XeOF4 + 2HF
(b) (i) In the group with increase in size of the element and increased bond distance, the bond dissociation energy decreases, therefore H-S bond dissociation energy is higher than H-Te and hence H-S bond breaks less easily than H-Te bond and H2S is a weaker acid than H2Te.
(ii) Because it contains two P-H bonds and thus reduces AgNO3 to metallic silver
4AgNO3 + H3PO2 + 2H2O → 4Agi ↓ + H3PO4 + 4HNO3
(iii) SO2 is a pungent and irritating gas. It acts as an air pollutant due to the following reasons:
- It causes throat and eye irritation as it is absorbed readily by respiratory tract.
- It combines with moisture forming sulphurous acid. It is then converted into H2SO4. Both these acids cause acid rain and destroy the marble, corrode metals, deteriorate fabrics, paper, leather etc.
Question 157.
Account for the following :
(a) Phosphorus shows high tendency for catenation.
(b) F2 is more reactive than ClF3 but ClF3 is more reactive than Cl2.
(c) Nitrogen is found in gaseous state.
(d) Decomposition of ozone molecule is a spontaneous process.
(e) SF6 is inert towards hydrolysis. (Comptt. Delhi 2013)
Answer:
(a) The bond strength of P-P is more tan N-N, therefore, phosphorous shows more tendency for catenation than nitrogen.
(b) Because the bond between Cl-F is weaker than the bond between Cl-Cl due to less effective overlapping of orbitals of Cl-F than Cl-Cl.
The F2 is most reactive due to its high electrode potential.
(c) Due to small size and high electronegativity nitrogen forms pπ – pπ multiple bonds, which are held together by weak Van-der Waal’s forces of attraction which can be easily broken. Hence N2exists as a gas at room temperature.
(d) Because O3 is thermodynamically unstable and its decomposition results in liberation of heat (ΔH is -ve) and increase in entropy (ΔS is +ve) so (ΔG becomes -ve and process becomes spontaneous.
(c) In SF6, S is protected by 6F atoms and does not allow H2O molecules to attach on it. So SF6 inerts towards hydrolysis.
Question 158.
(a) Complete the following chemical equations:
(i) P4 + NaOH + H2O →
(ii) XeF4 + O2F2 →
(b) How would you account for the following situations?
(i) The acidic strength of these
compounds increases in the following order :
PH3 > H2S > HCl
(ii) The oxidising power of oxoacids of chlorine follows the order :
HClO4 > HClO3 > HClO2 > HClO
(iii) In vapour state sulphur exhibits paramagnetic behaviour. (Comptt. Delhi 2014)
Answer:
(a)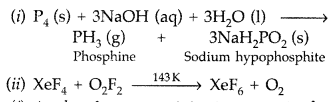
(b) (i) As the electronegativity increases in the same period from left to right so their electronegativity are in the increasing order, P < S < Cl.
In the same way the acid strength is also in the increasing order i.e. PH3 < H2S < HCl.![]()
Acidic strength of oxoacids of the same halogen increases with increase in oxidation number of the halogen because of the relative stability of the anions left after removal of proton. Thus as the number of oxygen atoms in the anion increases, the dispersal of the negative charge through pn-pn back bonding also increases and hence stability and acidic strength increases
(iii) In vapour state sulphur partly exists as S2molecule which has two unpaired electrons in the antibonding π orbitals and hence exhibits paramagnetism.
Question 159.
(a) Using VSEPR theory predict the probable structures of the following :
(i) N2O3 (ii) BrF3
(b) Arrange the following groups of substances in the order of the property indicated against each group :
(i) NH3, PH3, AsH3, SbH3 – increasing order of boiling points.
(ii) O, S, Se, Te – increasing order of electron gain enthalpy with negative sign.
(iii) F2, Cl2, Br2, I2 – increasing order of bond dissociation enthalpy. (Comptt. Delhi 2014)
Answer:
(a)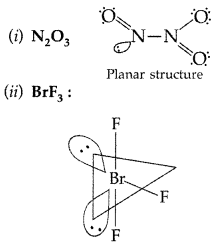
(b) (i) PH3 < AsH3 < NH3 < SbH3
(ii) O < Te < Se < S
(iii) The increasing order of bond dissociation enthalpy is :
Cl2 > Br2 > F2 > I2
Question 160.
(a) Write the formula and describe the structure of a noble gas species which is isostructural with
(i) IBr2 (ii) BrO3–
(b) Assign reasons for the following :
(i) SF6 is kinetically inert.
(ii) NF3 is an exothermic compound whereas NCl3is not.
(iii) HCl is a stronger acid than HF though flourine is more electronegative than chlorine. (Comptt. All India 2014)
Answer:
(a) (i) IBr2– : It has 2 bond pairs and 3 lone pairs.
Therefore according to VSEPR theory it should have Linear structure.
IBr2- has (7 + 2 × 7 + 1) i.e. 22 valence electrons.
No noble gas has 22 electrons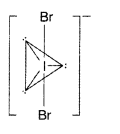
∴ The isoelectronic species for IBr2– is XeF2
(ii) BrO3–: It has 3 bond pairs and 1 lone pair of electrons. Therefore according to VSEPR theory it has pyramidal structure.
It has 26 valence electrons i.e. (7 + 3 × 6 + Pyramidal shape 1 = 26).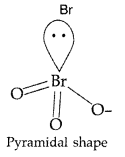
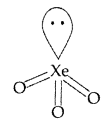
A noble gas having 26 valence electrons is XeO3 (8 + 3 × 6 = 26).
Thus XeO3 also has pyramidal structure.
(b) (i) Because SF6 is showing steric hindrance due to 6 (six) fluorine atoms which make it unable to react further with any other atom.
(ii)
- Because of enthalpy of dissociation of S- S bond is higher than 0-0 bond and the hydration energy of S2- is less than that of O2- ion.
- Due to smaller size of F as compared to Cl, the N – F bond is much stronger than N-Cl bond while bond dissociation energy of F2 is much lower than that of Cl2. Therefore, energy released during the formation of NF3molecule is more than the energy needed to break N2 and F2 molecules into individual atoms. In other words, formation of NF3 is an exothermic reaction.
The energy released during the formation of NCl3 molecule is less than the energy needed to break N2 and Cl2 molecule into individual atoms. Thus formation of NCl3 is an endothermic reaction.
(iii) Because HCl has large size and less bond strength than HF which makes easier liberation of H+.
Question 161.
(a) How is ammonia prepared on a large scale?
Name the process and mention the optimum conditions for the production of ammonia by this process.
(b) Assign reasons for the following :
(i) H2S is more acidic than H2O.
(ii) NH3 is more basic than PH3.
(iii) Sulphur has a greater tendency for catenation than oxygen. (Comptt. All India 2014)
Answer:
(a) According to Le Chatelier’s principle the favourable conditions for the production of NH3 by Haber’s process are
- pressure of 200 atmosphere
- temperature of ~ 700 K
- use of catalyst like Fe2O3 with small amount of K2O and Al3O3
N2 + 3H2 ⇌ 2NH3
(b) (i) H2S is more acidic than H20 because bond dissociation enthalpy of H – S bond in H2S is less than that of H – O bond in H2O.
(ii) Since both P and N contain lone pairs of electrons but due to small size and high electronegativity of Nitrogen in NH3, the electron density is much higher than PH2 therefore it can easily donate electrons and acts as strong Lewis base than PH2.
(iii) The greater catenation tendency of sulphur is due to two reasons :
- The lone pair of electrons feels more repulsion in O-O bond than S-S bond due to its small size and thus S – S forms strong bond.
- As the size of atom increases down the group from O-PO, the strength of bond increases and therefore catenation tendency also increases.
Question 162.
(a) Account for the following :
(i) Acidic character increases from HF to HI.
(ii) There is large difference between the melting and boiling points of oxygen and sulphur.
(iii) Nitrogen does not form pentahalide.
(b) Draw the structures of the following :
(i) ClF3 (ii) XeF4 (Delhi, All India 2015)
Answer:
(a) (i) Acidic character increases from HF to HI because bond dissociation enthalpy decreases from HF to HI.
(ii) Oxygen exists as diatomic O2 molecule while sulphur exists as polyatomic S8 molecule which has very high molecular mass therefore sulphur has much high melting and boiling points.
(iii) Nitrogen does not contain’d’ orbitals.
(b) (i) ClF3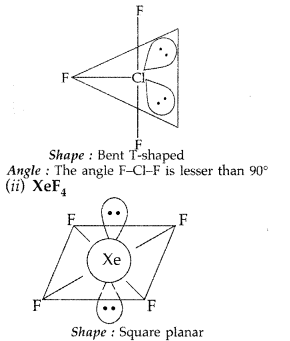
Question 163.
(i) Which allotrope of phosphorus is more reactive and why?
(ii) How the supersonic jet aeroplanes are responsible for the depletion of ozone layer?
(iii) F2 has lower bond dissociation enthalpy than Cl2. Why?
(iv) Which noble gas is used in filling balloons for meteorological observations?.
(v) Complete the equation :
XeF2 + PF5 → (Delhi, All India 2015)
Answer:
(i) White phosphorus is more reactive because it is less stable due to angular strain.
(ii) The oxides of nitrogen released by the exhausts of supersonic jetplanes are causing the depletion of ozone layer.
(iii) F2 has lower dissociation enthalpy as due to small size of F there is strong repulsive forces between F-F electrons.Bond dissociation enthalpy of F2 is less than that of Cl2 because of large electron-electron repulsion among the lone pairs in small size F2molecule.
(iv) Helium is filled in the balloons for meteorological observations.
(v) XeF2 + PF5 → [XeF]+ [PF]–
Question 164.
Account for the following :
(i) Acidic character increases from HF to HI.
(ii) There is large difference between the melting and boiling points of oxygen and sulphur.
(iii) Nitrogen does not form pentahalide.
Answer:
(i) Acidic character increases from HF to HI bond because dissociation enthalpy decreases from HF to HI due to which H+ ion releases easily.
(ii) Oxygen exists as diatomic molecule O2 while sulphur exists as S8 molecule which has very high molecular mass therefore sulphur has high BP and MP.
(iii) Nitrogen does not contain’d’ orbitals.
Question 165.
(a) Complete the following chemical equations :
(i) Cu + HNO3 (dilute) →
(ii) P4 + NaOH + H2O →
(b) (i) Why does R3P = O exist but R3N = O does not? (R = alkyl group)
(ii) Why is dioxygen a gas but sulphur a solid?
(iii) Why are halogens coloured? (Comptt. Delhi 2015)
Answer:
(a)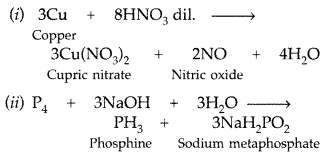
(b) (i) Nitrogen in R3N = O cannot form pπ – dπ multiple bonds because it cannot expand its covalency beyond 4 due to absence of d-orbital, so it will not exist. But R3P = O can do so due to presence of d-orbitals and formation of pπ – dπ multiple bonds which can expand its covalency up to 5.
(ii) Because of bigger size and the strong forces of attraction holding 8 atoms, their bonds cannot be broken easily and hence sulphur exists as solid while oxygen due to high electro-negativity and tendency to form pπ – dπ multiple bonds through Vander-waals forces of attraction can be broken easily and hence exists as gas.
(iii) All halogens are coloured due to absorption of light in the visible region as a result of which their electrons get excited to higher energy levels and while returning to lower level transmit energy of corresponding colour.
Question 166.
(a) Write balanced equations for the following reactions :
(i) Chlorine reacts with dry slaked lime.
(ii) Carbon reacts with concentrated H2SO4
(b) Describe the contact process for the manufacture of sulphuric acid with special reference to the reaction conditions, catalysts used and the yield in the process. (Comptt. Delhi 2015)
Answer:
(a)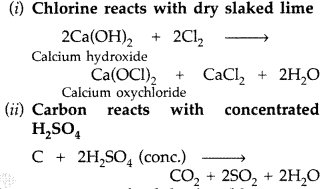
(b) Contact process of sulphuric acid :
It involves the following steps :
(i) Formation of sulphur dioxide by burning either sulphur or iron pyrites in excess of air.
S + O2 → SO2
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
(ii) Catalytic oxidation of SO2 into SO3 by using V2O5 as catalyst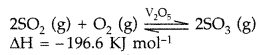
According to Le-Chatelier’s principle the reaction conditions are :
(a) High concentration of reactants
(b) Low temperature (623-723 K)
(c) High pressure (2 bar)
By obeying above conditions the yield of H2SO4will be 96 – 98%
(iii) Absorption of S03 in 98% H2S04 to give Oleum (H2S207)![]()
(iv) Dilution of Oleum to give sulphuric acid
H2S2O7 + H2O → 2H2SO4
Question 167.
(a) Elements of Gr. 16 generally show lower value of first ionization enthalpy compared to the corresponding periods of Gr. 15. Why?
(b) What happens when
(i) Concentrated H2SO4 is added to CaF2?
(ii) Sulphur dioxide reacts with chlorine in the presence of charcoal?
(iii) Ammonium chloride is treated with Ca(OH)2? (Comptt. All India 2015)
Answer:
(a) Elements of group 16, i.e., oxygen family have general electronic configuration of ns2np4 while elements of group 15, i.e., nitrogen family have general electronic configuration of ns2np3 which is a relatively stable half-filled configuration with high exchange energy and therefore require more ionization energy to release electrons from this stable configuration.
(b)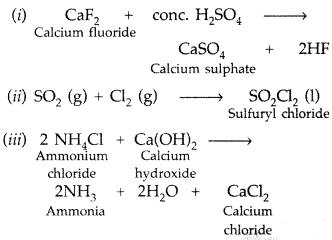
Question 168.
(a) Draw the structure of the following :
(i) BrF3 (ii) XeO3
(b) Answer the following :
(i) Why is NH2 more basic than PH3?
(ii) Why are halogens strong oxidising agents?
(iii) Draw the structure of XeOF4. (Comptt. All India 2015)
Answer:
(a)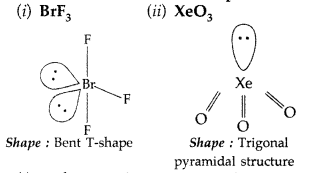
(b) (i) Both P and N contains lone pairs of electrons but due to small size and high electronegativity of nitrogen in NH3, the electron density is much higher than PH3, therefore it can easily donate electrons and acts as a strong Lewis base than PH3.
(ii) Halogens have strong tendency to accept an electron due to high negative electron gain enthalpies. Hence halogens act as strong oxidising agents.
(iii) XeOF4: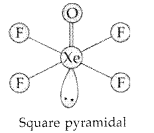
Question 169.
Account for the following:
(a) (i) Ozone is thermodynamically unstable.
(ii) Solid PCl5 is ionic in nature.
(iii) Fluorine forms only one oxoacid HOF.
(b) Draw the structure of (i) BrF5 (ii) XeF4 (Delhi 2016)
Answer:
(a) (i) Ozone is thermodynamically very unstable because:
• The decomposition of ozone into oxygen is exothermic in nature. (ΔH = -ve)
• There is also increase in entropy which in turn makes ΔG -ve and reaction spontaneous.
(ii) In solid state, PCl5 exists as [PCl3]+ [PCl6]– in which the cation is tetrahedral and anion is octahedral. Because of the presence of strong attractive forces, it is a solid.
(iii) Due to absence of d-orbitals in fluorine, it can only form one oxoacid i.e., HOF.
(b) (i) Structure of BrF5
Question 170.
(i) Compare the oxidizing action of F2 and Cl2 by considering parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy.
(ii) Write the conditions to maximize the yield of H2SO4 by contact process.
(iii) Arrange the following in the increasing order of property mentioned:
(a) H3PO3, H3PO4, H3PO2 (Reducing character)
(b) NH3, PH3, AsH3, SbH3, BiH3 (Base strength) (Delhi 2016)
Answer:
(i) Since the standard reduction potential value of fluorine is more than that of chlorine, so fluorine is a stronger oxidising agent.
- Bond dissociation enthalpy of F2 is less as compared to that of chlorine.
- The negative electron gain enthalpy of fluorine is slightly less than that of chlorine.
- The hydration enthalpy of flouride ion is much higher than that of Cl– ion due to smaller size.
(ii) Favourable conditions for manufacturing sulphuric acid by contact process are:
- high pressure of about 2 bars
- low temperature 720 K
- presence of V2O5 catalyst
(iii) (a) H3PO4 < H3PO3 < H3PO2 (Reducing character)
(b) BiH3 < SbH3 < AsH3 < PH3 < NH3 (Basic strength)
Question 171.
(a) What happens when :
(i) HCl reacts with finely powdered iron.
(ii) Cl3 reacts with hot concentrated solution of NaOH.
(b) Give appropriate reason for each of the following :
(i) Sulphur vapour exhibits some paramagnetic character.
(ii) NH3 is more basic than PH3
(iii) Dioxygen is a gas but sulphur a solid. (Comptt. Delhi 2016)
Answer:
(a) (i) It forms FeCl2 and H2 gas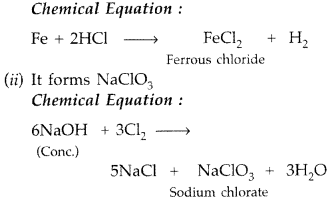
(b) (i) Sulphur above 1000 K exists partially as S2 molecule which has two unpaired
electrons in its Anti Bonding orbitals.
(ii) Due to small size of N, NH3 molecule has small surface area and high electron density in comparison to PH3. Hence it can donate lone pair of electron more readily.
(iii) In dioxygen there is Pπ – Pπ multiple bonding and no cation hence it is a gas.
Question 172.
(a) Complete the following equations :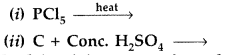
(b) Explain giving reason in each case :
(i) Halogens are strong oxidising agents
(ii) Fluorine form only one oxoacid, HOF
(iii) Helium is used in diving apparatus (Comptt. Delhi 2016)
Answer:
(a)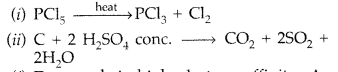
(b) (i) Due to their high electron affinity. As they have seven electrons, they accept one electron readily.
(ii) Due to high electronegativity and small size of fluorine.
(iii) Due to low solubility in blood.
Question 173.
Draw the structure of
(i) BrF3 (ii) XeOF4
Explain giving reason in each case :
(i) Why H2Te is more acidic than H2S?
(ii) Why are halogens strong oxidising agents?
(iii) Why does nitrogen show catenation tendency less than phosphorus? (Comptt. All India 2016)
Answer:
(a)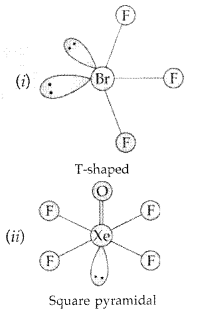
(b) (i) Because of low bond dissociation enthalpy of H-Te bond as Te has larger size than S.
(ii) Because of their strong electron affinity they have seven valence electrons.
(iii0 Because N-N single bond is weaker than P-P single bond.
Question 174.
(i) Why PCl5 gives fumes in moisture?
(ii) Why interhalogens are more reactive than pure halogens?
(b) Draw the structures of the following :
(i) PCl5(s) (ii)H2S2O8 (iii) XeF4 (Comptt. All India 2016)
Answer:
(a) (i) Because it reacts with moisture present in the air giving HCl.
(ii) Because of low bond dissociation enthalpy of interhalogens.
(b) (i) PCl5(s)
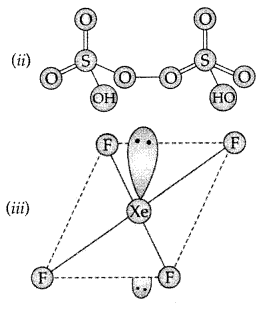
Question 175.
(a) When concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was evolved. This gas intensified when copper turnings were added to this test tube. On cooling the gas (A) changed into a colourless solid (B).
Identify (A) and (B). Write chemical reactions involved.
(b) Draw structure of XeOF4. (Comptt. All India 2017)
Answer:
(a) The salt is sodium nitrate which on heating with cone. H2SO4 evolves a brown gas i.e. NO2which gets intensified when Cu turnings are added.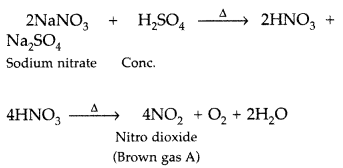
On cooling, NO2 condenses as a brown liquid which turns paler on cooling and eventually becomes a colourless solid due to formation of dimerized NO2 i.e. N2O4 (Dinitrogen tetraoxide).
A: Nitrogen dioxide (NO2)
B : Dinitrogen tetraoxide (Na2O4)
(b) Structure of XeOF4: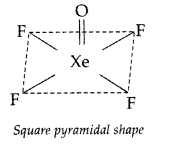
Question 176.
(a) Account for the following :
(i) Reducing character decreases from SO2 to TeO2.
(ii) HClO3 is a stronger acid than HCIO.
(iii) Xenon forms compounds with fluorine and oxygen only.
(b) Complete the following equations :
(i) 4NaCl + MnO2 + 4H2SO4 →
(ii) 6XeF4 + 12H2O → (Comptt. All India 2017)
Answer:
(a) (i) Reducing character decreases from SO2 to TeO2 because the pπ – pπ bonds in them become weaker with increase in size and bond length along the group.
(ii) HClO3 is a stronger acid than HClO because with increase in oxidation state and oxidation number the acidic character increases i.e. HClO3(+5) and HCIO (+1)
(iii) Xenon forms compounds with fluorine and oxygen only due to their high electronegativity and reactivity. The first ionisation energy of it is fairly close to that of O2 and F2.
(b) (i) 4NaCl + MnO2 + 4H2SO4 → 4NaHSO4 + MnCl2 + 2H2O + Cl2
(ii) 6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2
No comments:
Post a Comment