Important Questions
Question 1.
Give an example of linkage isomerism. (Delhi) 2010
Answer:
Linkage isomerism : When more than one atom in an ambidentate ligand is linked with central metal ion to form two types of complexes, then the formed isomers are called linkage isomers and the phenomenon is called linkage isomerism.
[Cr(H2O)5(NCS)]2+ Pentaaquathiocyanate chromium (III) ion
[Cr(H2O)5(NCS)]2+
Pentaaquaisothiocyanate chromium (III) ion
Question 2.
Give an example of coordination isomerism. (Delhi 2010)
Answer:
Example : [Co(NH3)6] [Cr(CN)6] and
[Cr(NH3)6] [CO(CN)6]
Give an example of ionization isomerism. (Delhi 2010)
Answer:
Example : [Pt (NH3)5 (Br)3] SO4 and
[Co (NH3)5 (SO4)] Br
Question 4.
Give IUPAC name of ionization isomer of [Ni(NH3)3NO3]Cl. (Comptt. All India 2012)
Answer:
IUPAC name : Triammine nitrato nickel (III) chloride
Question 5.
Write down the formula of : Tetraamineaquachloridocobalt(III) chloride. (Comptt. All India 2012)
Answer:
[Co(NH3)4(H2O)Cl]Cl2
Question 6.
Indicate the types of isomerisms exhibited by the complex [Co(NH3)5 (NO2)] (NO3)2. (At. no. Co = 27) (Comptt. All India 2012)
Answer:
It shows ionisation isomerism and linkage isomerism.
Question 7.
What type of bonding helps in stabilishing the a-helix structure of proteins? (Delhi 2013)
Answer:
a-helix formation -» Intramolecular hydrogen bonding.
Question 8.
Which complex ion is formed when undecomposed AgBr is washed with hypo solution in photography? (Comptt. All India 2013)
Answer:
Sodium dithiosulphato argentate (I) complex is formed![]()
Question 9.
Give IUPAC name of the ionization isomer of [Ni(NH3)3NO3]Cl. (Comptt. All India 2013)
Answer:
IUPAC name : Triammine chlorido nickel (II) nitrate [Ni(NH3)3NO3]Cl
Question 10.
Give two examples of ligands which form coordination compounds useful in analytical chemistry. (Comptt. All India 2013)
Answer:
Examples :
(i) EDTA (Ethylene diamine tetra-acetic acid)
(ii) Dimethyl glyoxime (DMG)
Question 11.
Which of the following is more stable complex and why?
[Co(NH3)6]3+ and [Co(en)3]3+ (Delhi 2014)
Answer:
[Co(en)3]3+ is more stable complex than [CO(NH3)6]3+ because of chelate effect.
Question 12.
What is the IUPAC name of the complex [Ni(NH3)6]Cl2? (Comptt. Delhi 2015)
Answer:
[Ni(NH3)6]Cl2
IUPAC name : Hexaamminenickel (II) chloride.
Question 13.
What is meant by chelate effect? (Comptt. All India 2015)
Answer:
Chelate effect : When a bidentate or a polydentate ligand contains donor atoms positioned in such a way that when they coordinate with the central metal ion, a five or a six membered ring is formed. This effect is called Chelate effect. As a result, the stability of the complex increases.
Example: the complex of Ni2+ with ‘+ion’ is more stable than NH3.
Question 14.
Write the IUPAC name of the following complex : [CO(NH3)6]3+ (Comptt. Delhi 2016)
Answer:
Hexaamminecobalt (III) ion.
Question 15.
Write the IUPAC name of the following coordination compound [NiCl4]2-. (Comptt. All India 2016)
Answer:
Tetrachloridonickelate (II) ion.
Question 16.
Why are low spin tetrahedral complexes not formed? (Comptt. Delhi 2017)
Answer:
Law spin tetrahedral complexes are rarely observed because orbital splitting energies for tetrahedral complexes are sufficiently large for forcing pairing.
Question 17.
Write IUPAC name of the complex: [CoCl2(en)2]+(Comptt. All India 2017)
Answer:
[CoCl2(en)2]+:
Dichloridobis(ethylenediamine)Cobalt(III)ion.
Question 18.
Write IUPAC name of the complex [Co(NH3)4Cl(NO2)]+. (Comptt. All India 2017)
Answer:
Tetra amminechloridonitro cobalt (III) ion.
Question 19.
Write IUPAC name of the complex: [CoCl2(en2)]+(Comptt. All India 2017)
Answer:
Dichloridobis ethylenediamine cobalt (III) ion.
Question 20.
Name the following coordination compounds according to IUPAC system of nomenclature :
(i) [Co(NH3)4 (H2O)Cl]Cl2
(ii) [CrCl2(en)2]Cl,
(en = ethane – 1, 2 – diamine) (Delhi 2010)
Answer:
(i) [CO(NH3)4 (H2O)Cl]Cl2
Tetraammine aquachlorido cobalt (III) chloride
(ii) [CrCl2(en)2]Cl
Dichlorobis (ethane-1, 2-diamine) chromium (III) chloride
Question 21.
Describe the shape and magnetic behaviour of following complexes :
(i) [CO(NH3)6]3+
(ii) [Ni(CN)4]2- (At. No. Co = 27, Ni = 28) (Delhi 2010)
Answer:
(i) [CO(NH3)6]3+ :
Orbitals of CO3+ ion :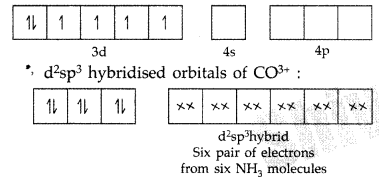
Hybridization : d2sp3 Shape : Octahedral Magnetic behaviour : Diamagnetic (absence of unpaired electrons)
(ii) [Ni(CN)4]2-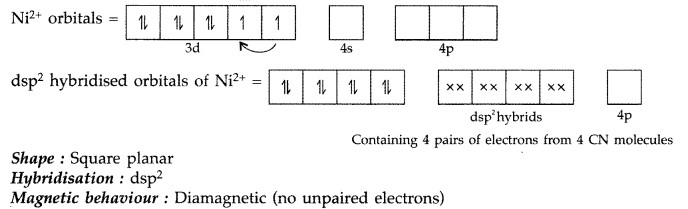
Question 22.
How is the stability of a co-ordination compound in solution decided ?
How is the dissociation constant of a complex defined? (Comptt. All India 2012)
Answer:
Stability of a complex in solution means the measure of resistance to the replacement of a ligand by some other ligand. This stablility can be expressed in terms of equilibrium constant.
Let the reaction between metal and ligand be represented as
Ma+ + nLx- ⇌ MLnb+
Stability or Dissociation constant (K)
=
The reciprocal of the stability constant K is known as instability constant or dissociation constant
Factors affecting the stability of a complex ion
(i) Nature of metal ion : Greater the charge and smaller the size of the ion, more is its charge density and greater will be stability of the complex.
(ii) Nature of ligand : More the basicity of ligand, more is its tendency to donate electron pair and therefore, more is the stability of the complex.
Question 23.
[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3- is weakly paramagnetic. Explain. (At. no. Fe = 26) (Comptt. All India 2012)
Answer:
In both the cases, Fe is in oxidation state +3. Outer electronic configuration of Fe+3 is :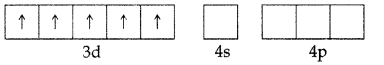
In the presence of CN–, the 3d electrons pair up leaving only one unpaired electron. The hybridisation involved is d2sp3 forming inner orbital complex which is weakly paramagnetic. In the presence of H2O (a weak ligand), 3d electrons do not pair up. The hybridisation involved is sp3d2forming an outer orbital complex. As it contains five unpaired electrons so it is strongly paramagnetic. .
Question 24.
Explain why [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex. (At. no. Co = 27, Ni = 28) (Comptt. All India 2013)
Answer:
In [Co(NH3)6]3+, the d-electrons of Co3+ ([Ar]3d645°) get paired leaving behind two empty d-orbital and undergo d2sp3 hybridization and hence inner orbital complex, while in [Ni(NH3)6]2+ the d-electrons of Ni2+ ([Ar]3d8 45°) do not pair up and use outer 4d subshell hence outer orbital complex.
Question 25.
Write the IUPAC name of the complex [Cr(NH3)4Cl2]+. What type of isomerism does it exhibit? (Delhi 2014)
Answer:
IUPAC name : Tetraamine dichlorido chromium (III) ion.
It exhibits geometrical isomerism.
Question 26.
(i) Write down the IUPAC name of the following complex :
[Cr(NH3)2CI3(en)]Cl (en = ethylenediamine)
(ii) Write the formula for the following complex : Pentaamminenitrito-o-Cobalt (III) (Delhi 2015)
Answer:
(I) [Cr(NH3)2Cl3(en)]Cl
IUPAC name : Diammine dichlorido ethylenediamine chromium (III) chloride.
(ii) [Co(NH3)5(ONO)]2+
Question 27.
(i) Write down the IUPAC name of the following complex :
[CO(NH3)5Cl]2+
(ii) Write the formula for the following complex : Potassium tetrachloridonickelate (II) (All India 2015)
Answer:
(i) [CO(NH3)5Cl]2+
IUPAC name : Pentaammine chlorido cobalt (III) ion
(ii) Formula of the complex potassium tetrachloridonickelate (II) K2[NiCl4]
Question 28.
When a co-ordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex.
(ii) IUPAC name of the complex. (Delhi 2016)
Answer:
(i) The complex formed on mixing a coordination compound CrCl3.6H2O with AgNO3 is as follows
CrCl3.6H2O + AgNO3 → [Cr(H2O5)Cl]Cl2. H2O
(ii) Pentaaquachloridochromium (III) chloride monohydrate
Question 29.
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3 moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex
(ii) IUPAC name of the complex (All India 2016)
Answer:
(i) Complex so formed is:
CoCl3.6NH3 + AgNO3 → [Co(NH3)6]Cl3
(ii) IUPAC name of complex is: Hexaamminecobalt (III) chloride
Question 30.
Using IUPAC norms write the formulae for the following:
(i) Sodium dicyanidoaurate (I)
(it) Tetraamminechloridonitrito-N-platinum (IV) sulphate (Delhi 2017)
Answer:
(i) Na[Au(CN)2]
(ii) [Pt(NH3)4 Cl(NO2)] (SO4)
Question 31.
Using IUPAC norms write the formulae for the following:
(a) Tris(ethane-1,2-diamine) chromium (III) chloride
(b) Potassium tetrahydroxozincate(II) (All India 2017)
Answer:
(a) [Cr(en)3] Cl3
(b) K2[Zn(OH)4]
Question 32.
Using IUPAC norms write the formulae for the following:
(a) Potassium trioxalatoaluminate (III)
(b) Dichloridobis(ethane-l, 2-diamine) cobalt (III) (All India 2017)
Answer:
(a) K3[Al(C2O4)3]
(b) [Co(Cl)2(en)2]+
Question 33.
For the complex [Fe(en)2Cl2], Cl, (en = ethylene diamine), identify
(i) the oxidation number of iron,
(ii) the hybrid orbitals and the shape of the complex,
(iii) the magnetic behaviour of the complex,
(iv) the number of geometrical isomers,
(v) whether there is an optical isomer also, and
(vi) name of the complex. (At. no. of Fe = 26) (Delhi 2009)
Answer:
(i) [Fe(en)2Cl2] Cl or x + 0 + 2 (-1) + (-1) = 0
x + (- 3) = 0 or x = + 3
∴ Oxidation number of iron, x = + 3
(ii) The complex has two bidentate ligands and two monodentate ligands. Therefore, the coordination number is 6 and hybridization will be d2sp3 and shape will be octahedral.
(iii) In the complex 26Fe3+ = 3d5 4s0 4p0![]()
Due to presence of one unpaired electrons in d orbitals the complex is paramagnetic.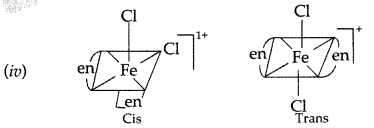
The number of geometrical isomers are two.
(v) In coordination complex of [Fe(en)2Cl2] Cl, only cis-isomer shows optical isomerism.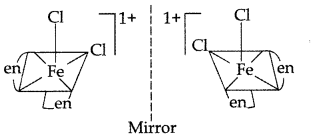
(vi) Name of complex: Dichloridobis (ethane-1, 2- diamine) Iron (III) chloride.
Question 34.
Compare the following complexes with respect to their shape, magnetic behaviour and the hybrid orbitals involved :
(i) [CoF4]2-
(ii) [Cr(H2O)2(C2O4)2]–
(iii) [Ni(CO)4] (Atomic number : Co = 27, Cr = 24, Ni = 28) (Delhi 2009)
Answer:
(i) [COF4]2_ : Tetrafluorido cobalt (III) ion
Coordination number = 4 Shape = Tetrahedral Hybridisation = sp3
Question 35.
Giving a suitable example for each, explain the following :
(i) Crystal field splitting
(ii) Linkage isomerism
(iii) Ambidentate ligand (All India 2009)
Answer:
(i) Crystal field splitting: It is the splitting of the degenerate energy levels due to the presence of ligands. When ligand approaches a transition metal ion, the degenerate d-orbitals split into two sets, one with lower energy and the other with higher energy. This is known as crystal field splitting and the difference between the lower energy set and higher energy set is known as crystal field splitting energy (CFSE)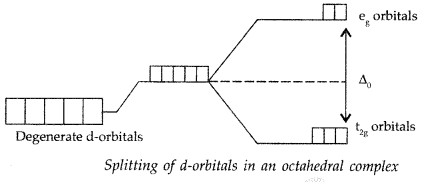
Example : 3d5 of Mn2+
(ii) Linkage isomerism: When more than one atom in an ambidentate ligand is linked with central metal ion to form two types of complexes, then the formed isomers are called linkage isomers and the phenomenon is called linkage isomerism.
[Cr(H2O)5(NCS)]2+ Pentaaquathiocyanate chromium (III) ion
[Cr(H2O)5(NCS)]2+
Pentaaquaisothiocyanate chromium (III) ion
(iii) Ambidentate ligand: The monodentate ligands with more than one coordinating atoms is known as ambidentate ligand. Monodentate ligands have only one atom capable of binding to a central metal atom or ion. For example, the nitrate ion NO2– can bind to the central metal atom/ion at either the nitrogen atom or one of the oxygen atoms.
Example : — SCN thiocyanate, — NCS isothiocyanate
Question 36.
Compare the following complexes with respect to structural shapes of units, magnetic behaviour and hybrid orbitals involved in units :
[Co(NH3)6]+3, [Cr(NH3)6]3+, Ni(CO)4
(At. nos. : Co = 27, Cr = 24, Ni = 28) (All India 2009)
Answer:
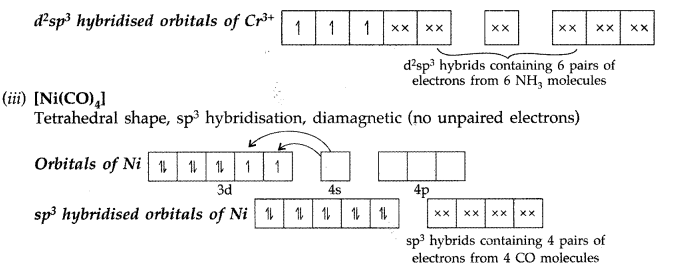
(i) [Co(NH3)6]+3 → Octahedral shape, d2sp3hybridisation, diamagnetic
Formation of [Co(NH2)6]+3 → oxidation state of Co is +3.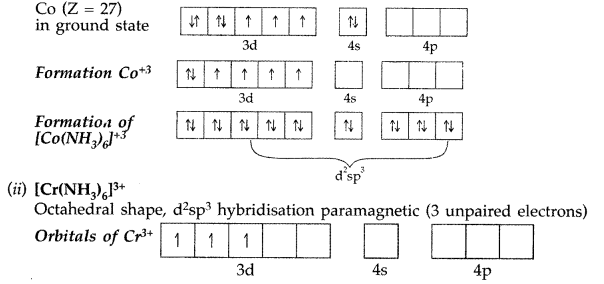
Question 37.
Explain the following :
(i) Low spin octahedral complexes of nickel are not known.
(ii) The π-complexes are known for transition elements only.
(iii) CO is a stronger ligand than NTL, for many metals. (All India 2009)
Answer:
(i) The electronic configuration of Ni is [Ar] 3d8 4s2which shows that it can only form two types of complexes i.e. square planar (dsp2) in presence of strong ligand and tetrahedral (sp3) in presence of weak ligand. There are four empty orbitals in Ni while octahedral complexes require six empty orbitals.
(ii) Due to presence of empty d-orbitals in transition metals, they can accept electron pairs from ligands containing π electrons and hence can form ic-bonding complexes.
Example : ligands like C5H5, C6H6 etc.
(iii) Due to greater magnitude of Δ0, CO produces strong fields which cause more splitting of d-orbitals and moreover it is also able to form π bond due to back bonding.
Question 38.
Compare the following complexes’ with respect to structural shapes of units, magnetic behaviour and hybrid orbitals involved in units :
(i) [Ni(CN)4]2- (ii) [NiCl4]2- (iii) [CoF6]3-
[At. Nos. : Ni = 28; Co = 27] (All India 2009)
Answer:
(i) [Ni(CN)4]2-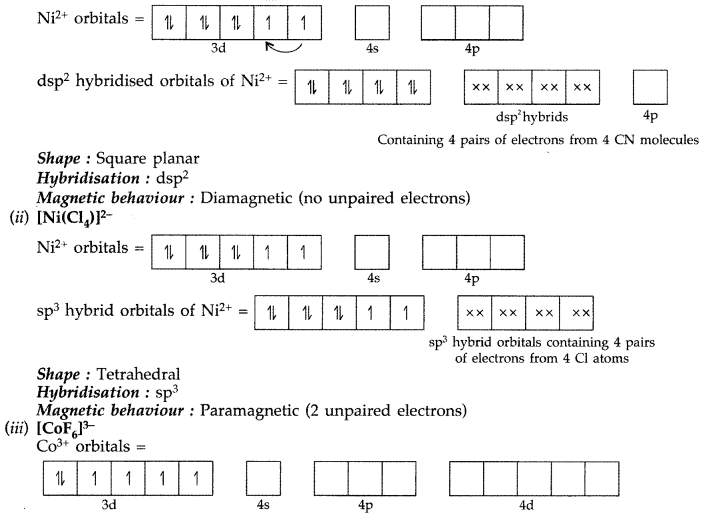
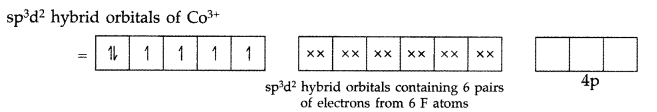
Shape : Octahedral outer orbital complex
Hybridisation : sp3d2
Magnetic behaviour : Paramagnetic (4 unpaired electrons)
Question 39.
Explain the following cases giving appropriate reasons :
(i) Nickel does not form low spin octahedral complexes.
(ii) The n-complexes are known for the transition metals only. (All India 2010)
Answer:
(i) The electronic configuration of Ni is [Ar] 3d8 4s2which shows that it can only form two types of complexes i.e. square planar (dsp2) in presence of strong ligand and tetrahedral (sp3) in presence of weak ligand. There are four empty orbitals in Ni while octahedral complexes require six empty orbitals.
(ii) Due to presence of empty d-orbitals in transition metals, they can accept electron pairs from ligands containing π electrons and hence can form ic-bonding complexes.
Example : ligands like C5H5, C6H6 etc.
(iii) Due to greater magnitude of Δ0, CO produces strong fields which cause more splitting of d-orbitals and moreover it is also able to form π bond due to back bonding.
Question 40.
Write the name, the state of hybridization, the shape and the magnetic behaviour of the following complexes :
[CoCl4]2-, [Ni(CN)4]2-, [Cr(H2O)2(C2O4)2]–
(At No. : Co = 27, Ni = 28, Cr = 240 (All India 2010)
Answer:
(i) [CoCl2]– :
Name – Tetra chlorido Cobalt (II) ion
Shape = Tetrahedral
Hybridization = sp3
Magnetic property = Paramagnetic
(ii) [Ni(CN)4]2-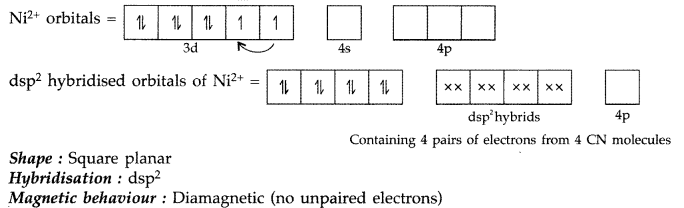
(iii) [Cr(H2O)2(C2O4)2]– :
Name = Diaquabis (oxalato) chromium (III) ion
Shape = Octahedral
Hybridization = d2sp3
Magnetic property = Paramagnetic
Question 41.
Write the name, stereochemistry and magnetic behaviour of the following : (At. nos. Mn = 25, Co = 27, Ni = 28) (Delhi 2011)
(i) K4[Mn(CN)6]
(ii) [CO(NH3)5 Cl]Cl2
(iii) K2 [Ni(CN)2]
Answer:
(i) K4[Mn(CN)2] : IUPAC name : Potassium Hexacyano manganate (II)
Geometry : Octahedral
Magnetic behaviour: Paramagnetic (one unpaired electron)
(ii) [CO(NH3)5 Cl]Cl2 :
Name : Pentaammine chlorido cobalt (III) chloride
Shape : Octahedral (∵ Coordination number = 6)
Hybridization : d2sp3 Magnetic behaviour : Diamagnetic (no unpaired electrons)
(iii) K2 [Ni(CN)4] :
Name : Potassium tetracyanonickelate (II)
Shape : Square planar Hybridization : dsp2 (∵ Coordination number = 4)
Hybridization : dsp2 Magnetic behaviour : Diamagnetic
Question 42.
Explain the following terms giving a suitable example in each case :
(i) Ambident ligand
(ii) Denticity of a ligand
(iii) Crystal field splitting in an octahedral field (All India 2011)
Answer:
(i) Ambidentate ligand : The monodentate ligands with more than one coordinating atoms is known as ambidentate ligand. Monodentate ligands have only one atom capable of binding to a central metal atom or ion. For example, the nitrate ion NO2– can bind to the central metal atom/ion at either the nitrogen atom or one of the oxygen atoms.
Example : — SCN thiocyanate, — NCS isothiocyanate
(ii) Denticity of a ligand: The number of donor atoms in a ligand which forms coordinate bond with the central metal atom are called denticity of a ligand.
Example : If donor atom is one then it is called Monodentate ligand, if it is two, then it is called Bidendentate and so on.
(iii) Crystal field splitting: It is the splitting of the degenerate energy levels due to the presence of ligands. When ligand approaches a transition metal ion, the degenerate d-orbitals split into two sets, one with lower energy and the other with higher energy. This is known as crystal field splitting and the difference between the lower energy set and higher energy set is known as crystal field splitting energy (CFSE)
Example : 3d5 of Mn2+
Question 43.
Write the structures and names of all the stereoisomers of the following compounds :
(i) [Co(en)3]Cl3
(ii) [Pt(NH3)2Cl2]
(iii) [Fe(NH3)4Cl2]Cl (All India 2011)
Answer:
(i) [Co(en)3]Cl3
Name ; Tris (ethane -1,2-diamine cobalt (III) chloride)
Hybridization : d2sp2 (∵ Coordination number = 6)
Shape : Octahedral
Magnetic behaviour : Diamagnetic
(ii) [Pt(NH3)2Cl2]
Name : Diammine dichlorido platinum (II) ion
Hybridization : dsp2( ∵ Coordination number = 4)
Shape : Square planar
Magnetic behaviour : Diamagnetic
(iii) [Fe(NH3)4Cl2]Cl
Name : Tetraammine dichlorido Iron (III) chloride
Hybridization : d2sp3 (∵ Coordination number = 6)
Shape : Octahedral
Magnetic behaviour diamagnetic :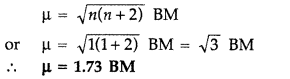
Question 44.
Write the state of hybridization, the shape and the magnetic behaviour of the following complex entities :
(t) [Cr(NH3)4Cl2]Cl
(ii) [Co(en)3]Cl3
(iii) K2[Ni(CN)4] (All India 2011)
Answer:
(i) [Cr(NH3)s4Cl2]Cl :
Hybridization : d2sp3
Shape : Octahedral
Magnetic behaviour: Paramagnetic
(ii) [Co(en)3] Cl3 :
Hybridization : d2sp3
Shape : Octahedral
Magnetic behaviour : Diamagnetic
(iii) K3[Ni(CN)4] :
Hybridization ; dsp2
Shape : Square planar
Magnetic behaviour: Diamagnetic
Question 45.
Give the formula of each of the following coordination entities :
(i) Co3+ ion is bound to one Cl–, one NH3 molecule and two bidentate ethylene diamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions.
Write the name and magnetic behaviour of each of the above coordination entities.
(At nos. Co = 27, Ni = 28) (Delhi 2011)
Answer:
(i) [Co (en)2 (NH3)Cl]2+ : amminechloridobis = (ethane-1, 2-diamine) cobalt (ID), “diamagnetic”
(ii) [Ni(C2O4)2 (H2O)2]-2 : diaquadioxalatonickelate (II), “paramagnetic”
Question 46.
State a reason for each of the following situations :
(i) Co2+ is easily oxidized to Co3+ in presence of a strong ligand.
(ii) CO is a stronger complexing reagent than NH3.
(iii) The molecular shape of [Ni(CO)4] is not the
same as that of [Ni(CN)4]2- (Delhi 2011)
Answer:
(i) Because in the presence of strong ligands, the crystal field splitting energy is more than the energy required to oxidise Co2+.
(ii) This is due to the formation of π – bond by back donation of electrons from metal to carbon of CO or due to synergic bonding.
(iii) CO is a stronger field ligand than CN. Ni is in zero oxidation state in Ni(CO)4 and has tetrahedral geometry. But, Ni is in +2 oxidation state in [Ni(CN)4]2- and has dsp2 hybridization (different geometry than tetrahedral sp3).
Question 47.
Write the name, the structure and the magnetic behaviour of each one of the following complexes :
(i) [Pt(NH3)2Cl(NO2)]
(ii) [Co(NH3)4Cl2]Cl
(iii) Ni(CO)4 (Atmos. Co = 27, Ni = 28, Pt = 78) (Delhi 2011)
Answer:
(i) [Pt(NH3)2Cl(NO2)]
Name : Diamine chloridonitroplatinum II
Structure :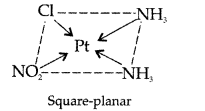
Magnetic behaviour: paramagnetic
(ii) [Co(NH3)4Cl2]Cl
Name : Tetraamminedichloridocobalt (III) chloride
Structure : octahedral
Magnetic behaviour : diamagnetic
(iii) Ni(CO)4
Name : Tetracarbonylnickel (O)
Structure : tetrahedral
Magnetic behaviour : diamagnetic.
Question 48.
Name the following coordination entities and draw the structures of their stereoisomers :
(i) [Co(en)2Cl2]+ (en = ethan-1, 2-diamine)
(ii) [Cr(C2O4)3]3-
(iii) [Co(NH3)3 Cl3] (Atomic numbers Cr = 24, Co = 27) (All India 2011)
Answer:
(i) [Co(en)2Cl2]+
Name : Dichlorido bis (en = ethan-1, 2-diamine) Cobalt (III)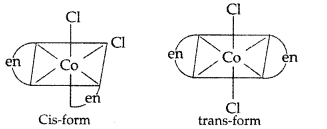
The geometrical isomers of [CoCl2(en)2]+ (2 isomers)
(ii) [Cr(C2O4)3]3-
Name : Trioxalatochromate (III) ion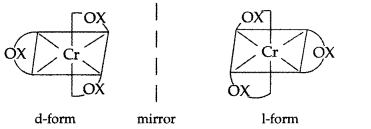
(iii) [Co(NH3)3 Cl3]
Name : Triamminetrichlorido cobalt (III)
Structure : The geometrical isomers of [Co(NH3)3Cl3] (2 isomers) :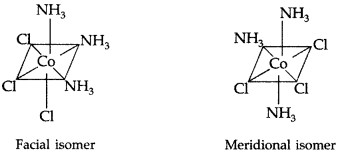
Question 49.
Name the following coordination entities and describe their structures :
(i) [Fe(CN)6]4-
(ii) [Cr(NH3)4Cl2]+
(iii) [Ni(CN)4]2-
(Atomic numbers Fe = 26, Cr = 24, Ni = 28) (All India 2011)
Answer:
(i) [Fe(CN)6]4-
Name : Hexacyanoferate (II) ion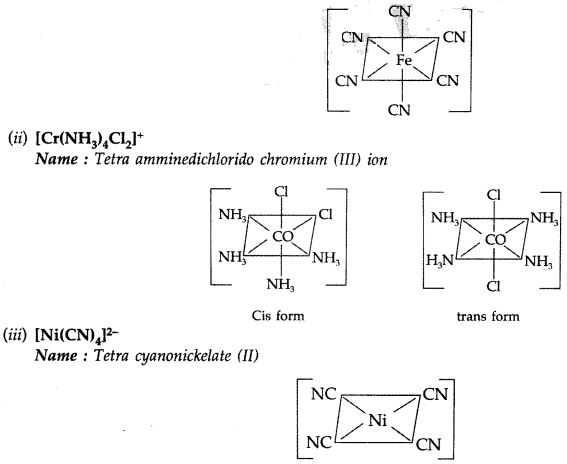
Question 50.
(a) Give two examples of coordination compounds used in industries.
(b) Using valence bond theory, explain the geometry and magnetic behaviour of [Co(NH3)6]3+
(At. no. of Co = 27) (Comptt. Delhi 2011)
Answer:
(a) Examples:
(i) Pure Ni can be obtained from Ni(CO)4
(ii) Gold and Ag are extracted by the use of complex formation like Na[Ag(CN)2].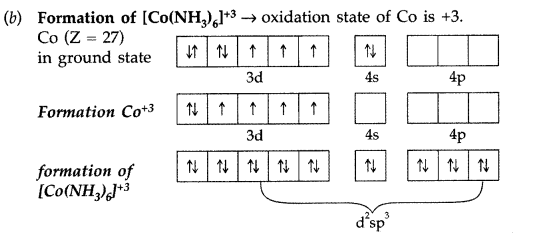
It involves d2sp3 hybridisation, Octahedral shape and diamagnetic due to absence of unpaired electrons.
Question 51.
(a) Give names of two complexes which are used in medicines.
(b) Using valence bond theory of complexes, explain the geometry and magnetic nature of [Ni(CN)4]2-. (At. no. of Ni = 28) (Comptt. Delhi 2011)
Answer:
(a) (i) Cis – platin[Pt(NH3)2Cl2] is used in the treatment of cancer.
(ii) EDTA is used in the treatment of lead poisoning.
(b) [Ni(CN)4]-2 The electronic configuration of Ni is 3d84s2.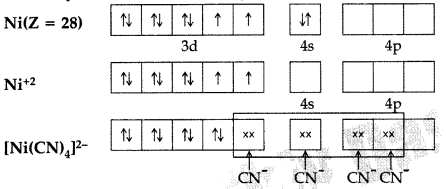
dsp2 hybridisation. The complex has square planar geometry and is diamagnetic in nature due to absence of unpaired electrons.
Question 52.
Write the IUPAC names of the following coordination compounds:
(i) [Cr(NH3)3Cl3]
(ii) K3[Fe(CN)6]
(iii) [CoBr2(en)2]+, (en = ethylenediamine) (Delhi 2013)
Answer:
(i) IUPAC name : [Cr(NH3)3Cl3]: Triammine trichlorido chromium (III)
(ii) IUPAC name : K3[Fe(CN)6]: Potassium hexacyanoferrate (III)
(iii) IUPAC name : [CoBr2(en)2]+: Dibromidobis (ethane-1,2-diamine) cobalt (III)
Question 53.
Write the types of isomerism exhibited by the following complexes:
(i) [Co(NH3)5CI]SO4
(ii) [Co(en)3]3+
(iii) [Co(NH3)6] [Cr(CN)6] (Delhi 2013)
Answer:
(i) [CO(NH3)5CI]SO4 — Ionisation isomerism
(ii) [Co(en)3]3+ — Optical isomerism
(iii) [Co(NH3)6][Cr(CN)6]— Coordination isomerism
Question 54.
For the complex [NiCl4]2-, write
(i) the IUPAC name
(ii) the hybridization type
(iii) the shape of the complex. (Atomic no. of Ni = 28) (All India 2013)
Answer:
(i) IUPAC name : [NiCl4]2- Tetrachloridonickelate (II) ion
(ii) Hybridization type : The above complex shows sp3 hybridization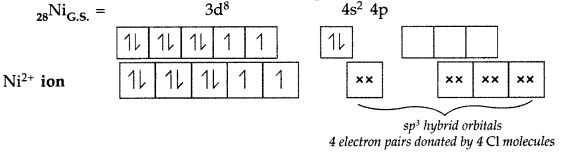
(iii) Shape : The above complex shows tetrahedral shape due to sp3 hybridization.
Question 55.
What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d4 in terms of t2g and eg in an octahedral field when
(i) Δ0 > P
(ii) Δ0 < P (All India 2013)
Answer:
Crystal field splitting energy : When ligands approach the central metal ion, the degenerate d-orbitals split into two sets, one with lower energy (t2g) and the other with higher energy (eg). The difference of energy between these two sets of orbitals is called crystal field splitting energy. (Δ0 for octahedral complexes).
The magnitude of Δ0 decides the actual configuration of d-orbitals by the help of mean pairing energy.
- If P > Δ0 then pairing of electrons does not occur and electrons enter in the higher energy e orbitals and thus form high spin complexes due to weak field ligands.
- If P < Δ0 then pairing of electrons occurs within the same set and form low spin complexes due to strong field ligands.
Question 56.
(a) How is a double salt different from a complex?
(b) Write IUPAC names of the following :
(i) K3[Fe(C2O4)3]
(ii) [Pt(NH3)6]Cl4.
(c) Draw the structure of cis isomer of [CO(NH3)4Cl2]+ (Comptt. Delhi 2013)
Answer:
(a) Double salt dissociates completely into its constituent ions in their aqueous solution.
Example : KCl.MgCl2.6H2O dissociates into K+, Cl–, Mg2+ and H2O
Complex does not dissociate into its constituent ions.
Example : K4[Fe(CN)6] → 4K+ + Fe(CN)6]4-
(b) (i) K3[Fe(C2O4)3] IUPAC name : Potassium trioxalatoferrate (III)
(ii) [Pt(NH3)6]Cl4 IUPAC name : Hexaammine Platinum (IV) chloride
(c) Structure of cis isomer of [CO(NH3)4Cl2]+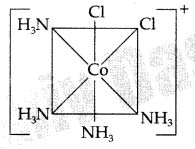
Question 57.
(a) Using Valence bond theory explain the geometry and magnetic behaviour by [Cr(NH3)6]3+. (At. no. Cr = 24)
(b) Write the IUPAC name of ionization isomer of [Ni(NH3)3NO3]Cl. (Comptt. Delhi 2013)
Answer:
(a) Cr atom (Z = 24), Ground state = [Ar] 3d5 4s1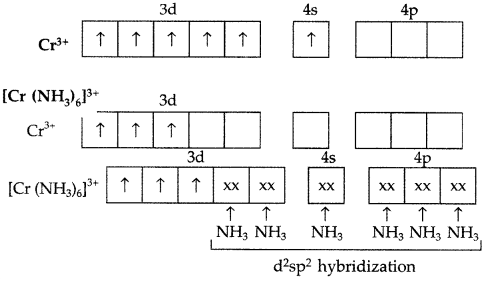
Geometry : Octahedral
Magnetic property : Paramagnetic
(b) IUPAC name : Triammine nitro nickel (II) Chloride
Question 58.
(i) Write the IUPAC name of the complex [Cr(NH3)4Cl2]Cl
(ii) What type of isomerism is exhibited by the complex {Co(en)3]3+?
(en = ethane-1, 2-diamine)
(iii) Why is [NiCl4]2- paramagnetic but [Ni(CO)4] is diamagnetic?
(At. nos. : Cr = 24, Co = 27, Ni = 28) (All India 2013)
Answer:
(i) IUPAC name : Tetraammine dichlorido chromium (III) chloride
(ii) Optical isomerism is exhibited by the complex [Co(en)3]3+
(iii) In [NiCl4]2-, Ni2+ has 3d84s0 configuration and due to weak ligand i.e. Cl–, electrons cannot pair up hence show paramagnetism while in [Ni(CO)4], Ni is in zero oxidation state with 3d84s2configuration and the 4s electrons are used up in pairing of 3d electrons as carbonyl ligand is strong hence diamagnetic.
Question 59.
Write down the IUPAC name for each of the following complexes:
(i) [CO(NH3)5Cl]Cl2
(ii) K3[Fe(CN)6]
(iii) [NiCl3]2- (Comptt. Delhi 2013)
Answer:
(i) [Co(NH3)3Cl] Cl2 : IUPAC name : Pentaammine chlorido cobalt (III) chloride
(ii) K3[Fe(CN)6] : IUPAC name : Potassium hexacyanoferrate (III)
(iii) [NiCl4]2-: IUPAC name : Petra chloridonickelate (II)
Question 60.
Draw the structures of optical isomers of each of the following complex ions:
[Cr(C2O4)3]3-, [PtCl2(en)2]2+, [Cr(NH3)2Cl2(en)]+(Comptt. Delhi 2013)
Answer:
Optical isomers of [Cr(C2O4)3]3-:
Name : Trioxalatochromate (III) ion
Optical isomers of [PtCl3(en)2]2+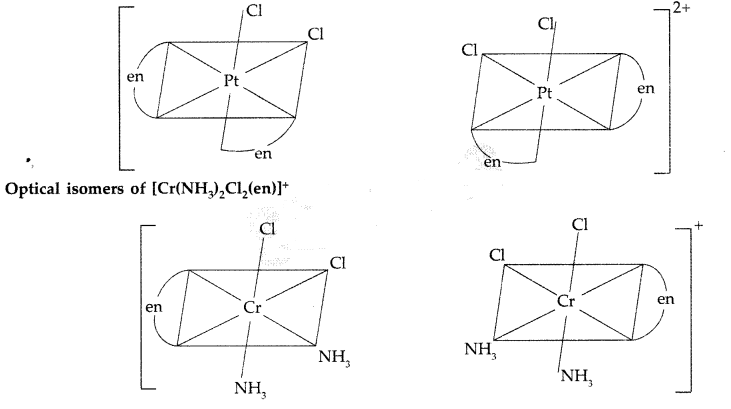
Question 61.
Write the IUPAC name and draw the structure of each of the following complex entities: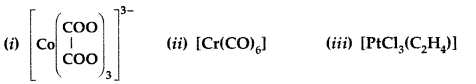
(At. nos. Cr = 25, Co = 27, Pt = 78) (Comptt. All India 2013)
Answer:
(i) IUPAC name : Trioxalato chromate (III) ion
(ii) IUPAC name : Hexa carbonyl chromium (o)
(iii) IUPAC name : Trichlorido ethylene platinum (II)
Question 62.
Giving one example in each of the following cases, discuss briefly the role of coordination compounds in
(i) extraction metallurgy of metals
(ii) analytical chemistry (Comptt. All India 2013)
Answer:
(i) Extraction metallurgy of metals : Gold and silver are extracted from their ores through formation of
cyanide complexes [Ag(CN)2]– and [(Au(CN)2]–respectively.
Example : Ag2S + 4NaCN ⇌ 2Na[Ag(CN)2] + Na2S
(ii) In analytical chemistry, they are used in qualitative analysis in which basic radicals are determined by converting them into suitable complexes with specific colour.
Example : Ni2+ is determined by DMG (Dimethyl Glyoxime) in the presence of NH4OH and forms a red ppt. of Ni DMG complex.
Similarly cobalt, Fe, Zn are also determined by converting them into complexes.
Question 63.
Write down the IUPAC names of the following complexes and also give stereochemistry and magnetic moment of the complexes :
(i) [Co(NH3)5Cl]Cl2
(ii) [CrCl3(py)3]
(iii) K4[Mn(CN)6]
(At. nos. Cr = 24, Mn = 25, Co = 27, py = pyridine) (Comptt. All India 2013)
Answer:
(i) [Co(NH3)5Cl]Cl2
IUPAC name : Pentaammine chlorido cobalt (III) chloride
C.N. of Co = 6 Shape = octahedral
o.s. of Co : x + 0 – 1 = +2 x = +3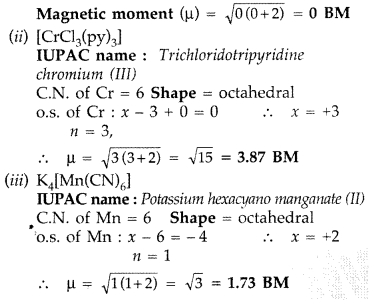
Question 64.
(i) Draw the geometrical isomers of complex [Pt(NH3)2Cl2].
(ii) On the basis of crystal field theory, write the electronic configuration for d4 ion if Δ0 < P.
(iii) Write the hybridization and magnetic behaviour of the complex [Ni(CO)4]. (At. no. of Ni = 28) (Delhi 2015)
Answer:
(ii) Electronic configuration for d4 ion if Δ0 < P is t2g3eg1 (high spin complex is formed)
(iii) Ni(CO)4 has sp3 hydbridization. It is diamagnetic in nature.
Question 65.
(i) What type of isomerism is shown by the complex [Cr(H2O)6]Cl3?
(ii) On the basis of crystal field theory, write the electronic configuration for d4 ion if Δ0 > P.
(iii) Write the hybridization and shape of [CoF6]3-.
(Atomic number of Co = 27) (All India 2015)
Answer:
(i) [Cr(H2O)6]Cl3 shows Hydration isomerism.
(ii) Electronic configuration for d4 ion if Δ0 > P is t2g4eg0
(iii) [CoF6]3- has sp3d2 hybridization and octahedral shape.
Question 66.
Indicate the types of isomerism exhibited by the following complexes :
(i) [CO(NH3)5(NO2)]2+
(ii) [Co(en)3]Cl3 (en = ethylene diamine)
(iii) [Pt(NH3)2Cl2] (Comptt. Delhi 2015)
Answer:
(i) Linkage isomerism is shown by [CO(NH3)5(NO2)]2+
[CO(NH3)5(NO2)]2+
Pentaamminenitro Cobalt (III)
[CO(NH3)2(O-NO)]2+
Pentaamminenitrito-N-Cobalt (III)
(ii) [Co(en)3]Cl3 shows optical isomerism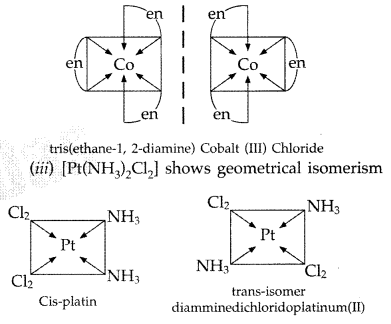
Question 67.
Write the IUPAC name of the following :
(i) [Co(NH3)6]Cl3
(ii) [NiCl4]2-
(iii) K3[Fe(CN)6] (Comptt. All India 2013)
Answer:
(i) [Co(NH3)6]Cl3
IUPAC name : Hexaammine cobalt (III) chloride.
(ii) [NiCl4]2-
IUPAC name : Tetrachlorido nickelate (II) ion.
(iii) K3[Fe(CN)6]
IUPAC name : Potassium hexacyano ferrate (III).
Question 68.
(a) For the complex [Fe(CN)6]3- write the hybridization type, magnetic character and spin nature of the complex. (At. number: Fe = 26).
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active. (Delhi 2016)
Answer:
(a) [Fe(CN)6]3-
The element Fe is in +3 oxidation state. As CN– ion is a strong field ligand, the electron pairing is possible in this case.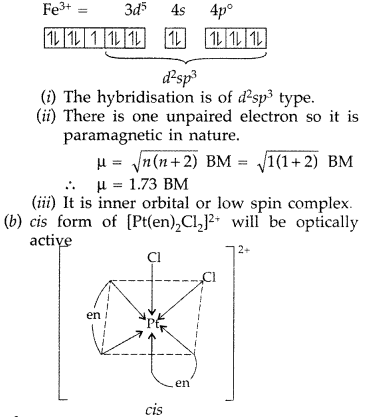
Question 69.
(a) For the complex [Fe(H2O)6]3+, write the hybridization, magnetic character and spin of the complex. (At. number: Fe = 26)
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive. (All India 2016)
Answer:
(a) [Fe(H2O)6]3+: The element Fe is in +3 oxidation state. As H2O is a weak field ligand, so electron pairing is not possible in this case.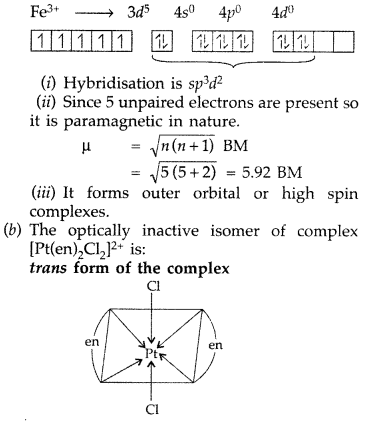
Question 70.
Write down the hybridization and magnetic character of the following complexes :
(i) [Ni(CO)4] (ii) [CoF6]-3 (Atomic number : Ni = 28, Co = 27) (Comptt. Delhi 2016)
Answer:
(i) Hybridisation : sp3
Magnetic character: Diamagnetic.
(ii) Hybridisation : sp3d2
Magnetic character : Paramagnetic.
Question 71.
Write the hybridization, shape and magnetic character of [Fe(CN)6]4-. (Comptt. All India 2016)
Answer:
Hybridisation : d2sp3
Shape : Octahedral
Magnetic character : Diamagnetic as all the electrons get paired due to strong field ligand CN. Fe(II)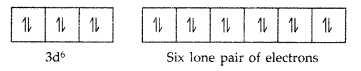
Question 72.
(i) What type of isomerism is shown by the complex [Co(NH3)6][Cr(CN)6]?
(ii) Why a solution of [Ni(H2O)6]2+ is green while a solution of [Ni(CN)4]2- is colourless? (At. no. of Ni = 28)
(iii) Write the IUPAC name of the following complex: [CO(NH3)5(CO3)]Cl. (Delhi 2017)
Answer:
(i) Coordination isomerism
(ii) [Ni(H2O)6]2+ is an outer orbital complex due to weak field ligand H2O and the presence of unpaired electrons undergoes d—d transition by absorbing red light and shows green colour while [Ni(CN)4]2- is an inner orbital complex and has no unpaired electrons hence colourless.
(iii) Pentaamminecarbonatocobalt (III) Chloride
Question 73.
(i) What type of isomerism is shown by the complex [Co(en)3]Cl3?
(ii) Write the hybridisation and magnetic character of [Co(C2O4)3]3-.
(At. no. of Co = 27)
(iii) Write IUPAC name of the following complex: [Cr(NH3)3Cl3] (Delhi 2017)
Answer:
(i) [Co(en)3]Cl3 show’s optical isomerism.
(ii) [Co(C2O4)3]3- shows d2sp3 hybridisation and is diamagnetic in nature.
(iii) IUPAC name: Triamminetrichloridochromium(III).
Question 74.
(a) What type of isomerism is shown by the complex [Co(NH3)5 (SCN)]2+?
(b) Why is [NiCl24]2- paramagnetic while [Ni(CN)4]2-is diamagnetic? (Atomic number of Ni = 28)
(c) Why are low spin tetrahedral complexes rarely observed? (All India 2017)
Answer:
(a) [Co(NH3)5 (SCN)]2+ shows linkage isomerism.
(b) Since in [NiCl4]2- Cl– is a weak field ligand, it forms outer orbital complex and has unpaired electrons which imparts paramagnetic character to complex while in [Ni(CN)4]2-, CN– is a strong field ligand, forms inner orbital complex and has paired electrons which imparts diamagnetic character to it.
(c) Low spin tetrahedral complexes are rarely observed because orbital splitting energies for tetrahedral complexes are not sufficiently large for forcing pairing.
Question 75.
For the complex ion [CoF6]3- write the hybridization type, magnetic character and spin nature. [Atomic number: Co = 27] (Comptt. Delhi 2017)
Answer:
[CoF6]3-
Co3+ = [Ar] 3d6 4s0 4p6 .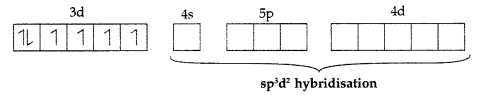
It is paramagnetic due to presence of 4 unpaired electrons and form high spin complex.
Question 76.
For the complex ion [Ni(CN)4]2- write the hybridization type, magnetic character and spin nature. [Atomic No.: Ni = 28] (Comptt. Delhi 2017)
Answer:
[Ni(CN)4]2-
Ni2+ = [Ar] 3d8 4s0 4p0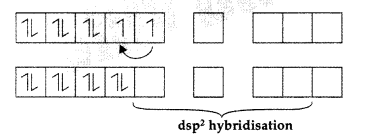
∴ Diamagnetic due to paired electrons. Complex is low spin.
Question 77.
For the complex ion [Fe(en)2Cl2]+ write the hybridization type and magnetic behaviour. Draw one of the geometrical isomer of the complex ion which is optically active. [Atomic No.: Fe = 26] (Comptt. All India 2017)
Answer:
In the complex of 26Fe3+ = 3d54s0 4p0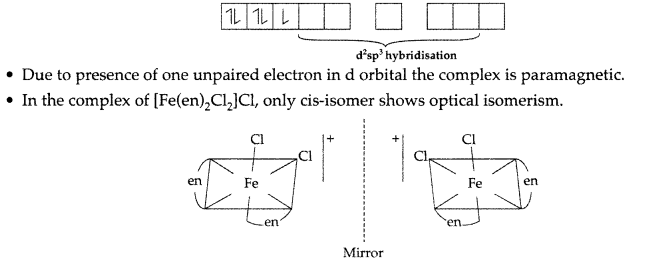
Question 78.
For the complex ion [CoCl2(en)2]+ write hybridization type and spin behaviour. Draw one of the geometrical isomers of the complex ion which is optically active. [Atomic No.: Co = 27] (Comptt. All India 2017)
Answer:
In the complex [CoCl2(en)2]+, 27Co3+ = [Ar]3d64s04p0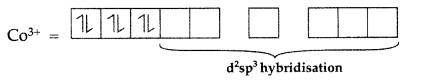
It is low spin complex.
Since inner d-orbitals are involved so it is an inner orbital complex.
In this complex only cis-isomer shows optical isomerism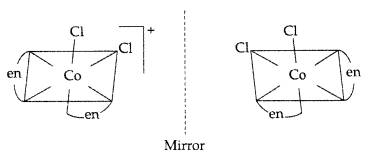
Question 79.
When a coordination compound CrCl3.6H2O is mixed with AgNO3 solution, 3 moles of AgCl are precipitated per mole of the compound. Write :
(i) Structural formula of the complex
(ii) IUPAC name of the complex
(iii) Magnetic and spin behaviour of the complex (Comptt. All India 2017)
Answer: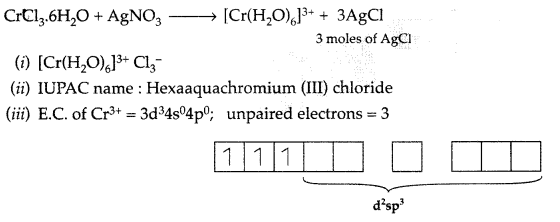
Inner orbital complex so it is low spin complex.
Since 3 unpaired electrons are present, it is paramagnetic in nature.
No comments:
Post a Comment