Occurrence and Extraction
Aluminium occurs widely as a constituent of rocks and soils.
The main ores of aluminium are :
Bauxite : Al2O3.2H2O
Cryolite : Na3AlF6
Feldspar: KAlSi3O8
Mica : KAlSi2O10(OH)2
In India, aluminium occurs mostly in the form of mica. The ores of mica are located in the states of Bihar, Tamil Nadu, Madhya Pradesh, Jammu and Kashmir.
Aluminium is normally extracted from bauxite ore, Al2O3. 2H2O. It involves two steps:
1) Purification of bauxite
2) Electrolysis of alumina.
1) Purification of Bauxite
The purification of bauxite ore can be done by Baeyer’s process.
a) The powdered bauxite ore is treated with hot concentrated (45%) solution of sodium hydroxide at 473-523 K and 35- 36 bar pressure.
b) In this process, alumina (Al2O3) dissolves forming sodium aluminate. This process is called leaching.
Al2O3.2H2O (s) + 2NaOH (aq) + H2O (l) ——> 2 Na[Al(OH)4] (aq)
Al2O3 +2 OH¯ + 3 H2O —–> 2[Al(OH)4]¯ (aq)
The impurities of ferric oxide and silica are insoluble and are removed by filtration.
The solution containing sodium aluminate is neutralised by passing CO2 , gas and hydrated Al2O3 is precipitated. At this stage, the solution is seeded with freshly prepared samples of hydrated Al2O3 which induces the precipitation.
2Na [Al(OH)4](aq) + CO2 (g) ——> Al2O3.xH2O (s) + 2 NaHCO3(s)
Some sodium silicate formed above remains in the solution and hydrated alumina is removed by filtration and is dried. It is heated to 1473 K to get pure alumina (Al2O3).
Al2O3.xH2O (s) —-> Al2O3 (s) + x H2O (g)
2) Electrolysis of fused Alumina
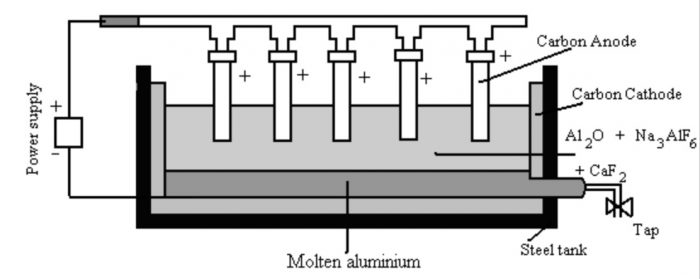
a) The alumina is dissolved in molten cryolite and is electrolysed in an iron tank lined inside with carbon.
b) The molten cryolite (Na3AlF6) decreases the melting point to about 1173 K and also increases the electrical conductivity.
c) The process of electrolysis is carried in an iron tank having a lining of carbon which acts as the cathode.
d) The anode consists of a number of carbon rods which dip in the fused electrolyte.
e) The electrolyte is covered with a layer of powdered coke. The overall reaction
2 Al2O3 + 3C —>4 Al + 3 CO2
This process of electrolysis is called Hall Heroult process.
During electrolysis the following reactions occur:
At cathode : Al3+ (melt) + 3e¯ ——> Al(l)
At anode : C(s) + O2- (melt) ——>CO (g) + 2e¯
Therefore, aluminium is liberated at the cathode and gets collected at the bottom of tank, from where it is removed periodically. The oxygen evolved at the anode combines with the carbon of the anode to produce carbon monoxide.
CO either burns to CO2 or escapes out.
3) Refining of Aluminium
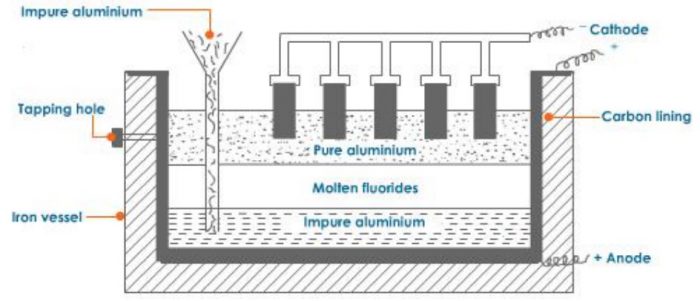
The aluminium metal obtained by the electrolysis of alumina is 99%% pure. It can be further refined by Hoop’s electrolytic method. The process in carried out in an iron tank lined inside with carbon. It has three layers of molten liquids having different densities.
1) The top layer consists of pure aluminium having carbon electrodes dipping in it. The carbon electrodes serve as cathode.
2) The middle layer has mixture of fluorides of sodium, barium and aluminium in the molten state. This acts as an electrolyte.
3) The bottom layer consists of impure aluminium which along with the carbon lining acts as the anode.
On passing electric current, aluminium ions from the middle layer are discharged at the cathode as pure aluminium. The pure aluminium is removed from the tapping hole. An equivalent amount of aluminium from the bottom layer moves into the middle layer leaving behind the impurities. Thus, this method gives completely pure aluminium.